当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dual Roles of Li3N as an Electrode Additive for Li‐Excess Layered Cathode Materials: A Li‐Ion Sacrificial Salt and Electrode‐Stabilizing Agent
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2018-08-20 , DOI: 10.1002/chem.201801809
Xiaofei Bian 1 , Qiang Pang 1 , Yingjing Wei 1 , Dong Zhang 1 , Yu Gao 1 , Gang Chen 1, 2
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2018-08-20 , DOI: 10.1002/chem.201801809
Xiaofei Bian 1 , Qiang Pang 1 , Yingjing Wei 1 , Dong Zhang 1 , Yu Gao 1 , Gang Chen 1, 2
Affiliation
|
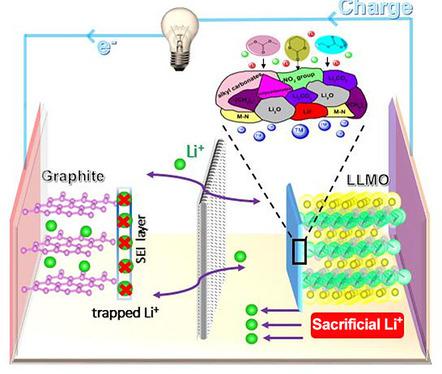
Li2CO3‐passivated Li3N with high stability is prepared by aging Li3N powder in dry air, and is then used as an electrode additive for a Li(Li0.18Ni0.15Co0.15Mn0.52)O2 (LLMO) cathode material. The material shows a large irreversible capacity of 800 mA h g−1 during the first charge, with the formation of a Li2N intermediate product. Acting as a Li+ sacrificial salt for a LLMO(+)/graphite(−) Li‐ion battery, 2 wt % Li3N results in a 10 % increase in discharge capacity. The Li2N intermediate product reacts with the electrolyte, forming a uniform and regular surface film on the cathode. Moreover, chemical bonding between LLMO and N improves the electrode stability, resulting in excellent electrochemical performance.
中文翻译:

Li3N作为过量锂分层电极材料的电极添加剂的双重作用:锂离子牺牲盐和电极稳定剂
通过在干燥空气中老化Li 3 N粉末来制备具有高稳定性的Li 2 CO 3钝化的Li 3 N,然后将其用作Li(Li 0.18 Ni 0.15 Co 0.15 Mn 0.52)O 2(LLMO)的电极添加剂阴极材料。该材料在首次充电期间显示出800 mA h g -1的不可逆大容量,并形成了Li 2 N中间产物。2 wt%的Li 3 N充当LLMO(+)/石墨(-)锂离子电池的Li +牺牲盐,导致放电容量增加10%。李2N中间产物与电解质反应,在阴极上形成均匀且规则的表面膜。此外,LLMO和N之间的化学键提高了电极的稳定性,从而获得了出色的电化学性能。
更新日期:2018-08-20
中文翻译:

Li3N作为过量锂分层电极材料的电极添加剂的双重作用:锂离子牺牲盐和电极稳定剂
通过在干燥空气中老化Li 3 N粉末来制备具有高稳定性的Li 2 CO 3钝化的Li 3 N,然后将其用作Li(Li 0.18 Ni 0.15 Co 0.15 Mn 0.52)O 2(LLMO)的电极添加剂阴极材料。该材料在首次充电期间显示出800 mA h g -1的不可逆大容量,并形成了Li 2 N中间产物。2 wt%的Li 3 N充当LLMO(+)/石墨(-)锂离子电池的Li +牺牲盐,导致放电容量增加10%。李2N中间产物与电解质反应,在阴极上形成均匀且规则的表面膜。此外,LLMO和N之间的化学键提高了电极的稳定性,从而获得了出色的电化学性能。

































 京公网安备 11010802027423号
京公网安备 11010802027423号