当前位置:
X-MOL 学术
›
Adv. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ionizable Amino-Polyesters Synthesized via Ring Opening Polymerization of Tertiary Amino-Alcohols for Tissue Selective mRNA Delivery.
Advanced Materials ( IF 27.4 ) Pub Date : 2018-07-05 , DOI: 10.1002/adma.201801151
Piotr S Kowalski 1, 2 , Umberto Capasso Palmiero 1, 3 , Yuxuan Huang 1 , Arnab Rudra 1, 2 , Robert Langer 1, 2, 4, 5 , Daniel G Anderson 1, 2, 4, 5
Advanced Materials ( IF 27.4 ) Pub Date : 2018-07-05 , DOI: 10.1002/adma.201801151
Piotr S Kowalski 1, 2 , Umberto Capasso Palmiero 1, 3 , Yuxuan Huang 1 , Arnab Rudra 1, 2 , Robert Langer 1, 2, 4, 5 , Daniel G Anderson 1, 2, 4, 5
Affiliation
|
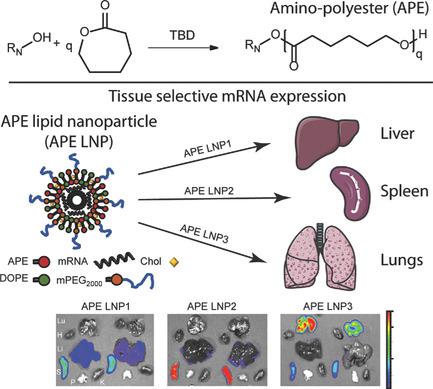
The utility of messenger RNA (mRNA) as a therapy is gaining a broad interest due to its potential for addressing a wide range of diseases, while effective delivery of mRNA molecules to various tissues still poses a challenge. This study reports on the design and characterization of new ionizable amino-polyesters (APEs), synthesized via ring opening polymerization (ROP) of lactones with tertiary amino-alcohols that enable tissue and cell type selective delivery of mRNA. With a diverse library of APEs formulated into lipid nanoparticles (LNP), structure-activity parameters crucial for efficient transfection are established and APE-LNPs are identified that can preferentially home to and elicit effective mRNA expression with low in vivo toxicity in lung endothelium, liver hepatocytes, and splenic antigen presenting cells, including APE-LNP demonstrating nearly tenfold more potent systemic mRNA delivery to the lungs than vivo-jetPEI. Adopting tertiary amino-alcohols to initiate ROP of lactones allows to control polymer molecular weight and obtain amino-polyesters with narrow molecular weight distribution, exhibiting batch-to-batch consistency. All of which highlight the potential for clinical translation of APEs for systemic mRNA delivery and demonstrate the importance of employing controlled polymerization in the design of new polymeric nanomaterials to improve in vivo nucleic acid delivery.
中文翻译:

通过叔氨基醇开环聚合合成的可电离氨基聚酯用于组织选择性 mRNA 递送。
信使 RNA (mRNA) 作为一种治疗方法的应用因其治疗多种疾病的潜力而受到广泛关注,但将 mRNA 分子有效递送至各种组织仍然是一个挑战。本研究报告了新型可电离氨基聚酯 (APE) 的设计和表征,该聚酯通过内酯与叔氨基醇的开环聚合 (ROP) 合成,能够实现组织和细胞类型选择性递送 mRNA。通过将多种 APE 库配制为脂质纳米粒子 (LNP),建立了对于有效转染至关重要的结构活性参数,并鉴定出 APE-LNP 可以优先归巢并引发有效的 mRNA 表达,且在肺内皮、肝脏中的体内毒性较低。肝细胞和脾抗原呈递细胞,包括 APE-LNP,其向肺部的全身 mRNA 递送能力比 vivo-jetPEI 强近十倍。采用叔氨基醇引发内酯的ROP可以控制聚合物分子量并获得分子量分布窄的氨基聚酯,并且表现出批次间的一致性。所有这些都凸显了 APE 临床转化用于全身 mRNA 递送的潜力,并证明了在新型聚合物纳米材料设计中采用受控聚合来改善体内核酸递送的重要性。
更新日期:2018-07-05
中文翻译:

通过叔氨基醇开环聚合合成的可电离氨基聚酯用于组织选择性 mRNA 递送。
信使 RNA (mRNA) 作为一种治疗方法的应用因其治疗多种疾病的潜力而受到广泛关注,但将 mRNA 分子有效递送至各种组织仍然是一个挑战。本研究报告了新型可电离氨基聚酯 (APE) 的设计和表征,该聚酯通过内酯与叔氨基醇的开环聚合 (ROP) 合成,能够实现组织和细胞类型选择性递送 mRNA。通过将多种 APE 库配制为脂质纳米粒子 (LNP),建立了对于有效转染至关重要的结构活性参数,并鉴定出 APE-LNP 可以优先归巢并引发有效的 mRNA 表达,且在肺内皮、肝脏中的体内毒性较低。肝细胞和脾抗原呈递细胞,包括 APE-LNP,其向肺部的全身 mRNA 递送能力比 vivo-jetPEI 强近十倍。采用叔氨基醇引发内酯的ROP可以控制聚合物分子量并获得分子量分布窄的氨基聚酯,并且表现出批次间的一致性。所有这些都凸显了 APE 临床转化用于全身 mRNA 递送的潜力,并证明了在新型聚合物纳米材料设计中采用受控聚合来改善体内核酸递送的重要性。































 京公网安备 11010802027423号
京公网安备 11010802027423号