当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxidation of Magnesium: Implication for Aging and Ignition
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2016-01-06 00:00:00 , DOI: 10.1021/acs.jpcc.5b08848
Hongqi Nie 1 , Mirko Schoenitz 1 , Edward L. Dreizin 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2016-01-06 00:00:00 , DOI: 10.1021/acs.jpcc.5b08848
Hongqi Nie 1 , Mirko Schoenitz 1 , Edward L. Dreizin 1
Affiliation
|
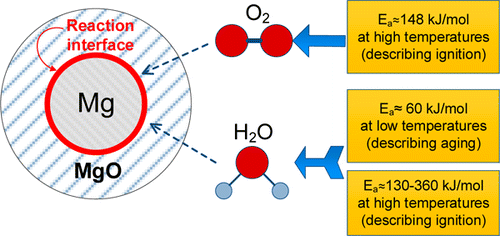
Magnesium is widely used in pyrotechnic formulations; it is also a component of reactive alloys, e.g., Al—Mg and B—Mg, which are potential fuels for explosives and propellants. Despite its widespread applications, the kinetics of oxidation of Mg powders are not well quantified. Such kinetics are of fundamental importance for the models aimed to describe thermally induced ignition of metal powders. In addition, for Mg the issue of aging is important because its oxide, MgO, is porous. In this work, magnesium oxidation by both oxygen and steam was studied by thermo-analytical measurements for micron-sized spherical powders. Heat flow calorimetry and thermogravimetric analysis were used to quantify reaction rates for low and elevated temperature ranges, respectively. Experiments with spherical powders with different but overlapping particle size distributions were used to identify the location of the reaction interface. The reaction was found to occur at the interface of metal and the growing oxide layer for all oxidizing conditions. The reaction is thus rate limited by diffusion of oxidizer to the metal surface. Kinetics of oxidation for both dry and humid oxidizing environments were quantified using thermo-analytical measurements and different data processing techniques. The activation energy of magnesium oxidation in humid environments at low temperatures is close to 60 kJ/mol. Activation energy for oxidation of magnesium in oxygen is 148 kJ/mol. For oxidation of magnesium in steam at elevated temperatures, the activation energy increases linearly from approximately 130 to 360 kJ/mol, while the thickness of the oxide layer is growing up to 2.4 μm. Simplified diffusion-limited reaction models were developed for oxidation of magnesium in both oxygen and steam. The models enable one to predict both preignition reactions occurring upon heating of Mg particles and the time of Mg powder aging when exposed to moisture or oxygen at different temperatures.
中文翻译:

镁的氧化:对衰老和着火的影响
镁广泛用于烟火配方中。它也是反应性合金的成分,例如Al-Mg和B-Mg,它们是爆炸物和推进剂的潜在燃料。尽管得到了广泛的应用,但是镁粉末的氧化动力学还没有得到很好的量化。这样的动力学对于旨在描述金属粉末的热诱导点火的模型至关重要。另外,对于镁来说,老化的问题很重要,因为其氧化物氧化镁是多孔的。在这项工作中,通过对微米级球形粉末进行热分析测量,研究了氧气和蒸汽对镁的氧化作用。热流量热法和热重分析法分别用于量化低温和高温范围的反应速率。使用具有不同但重叠的粒度分布的球形粉末进行的实验来确定反应界面的位置。对于所有氧化条件,发现反应发生在金属和生长的氧化物层的界面处。因此,反应速度受到氧化剂向金属表面扩散的限制。使用热分析测量和不同的数据处理技术,可以对在干燥和潮湿氧化环境下的氧化动力学进行定量。在潮湿的环境中,低温下镁氧化的活化能接近60 kJ / mol。氧气中镁的氧化活化能为148 kJ / mol。为了在高温下氧化蒸汽中的镁,活化能从大约130 kJ / mol线性增加到360 kJ / mol,而氧化层的厚度增长到2.4μm。针对氧气和蒸汽中镁的氧化,开发了简化的扩散受限反应模型。该模型使人们能够预测在加热Mg颗粒时发生的提前点火反应以及在不同温度下暴露于湿气或氧气时Mg粉末老化的时间。
更新日期:2016-01-06
中文翻译:

镁的氧化:对衰老和着火的影响
镁广泛用于烟火配方中。它也是反应性合金的成分,例如Al-Mg和B-Mg,它们是爆炸物和推进剂的潜在燃料。尽管得到了广泛的应用,但是镁粉末的氧化动力学还没有得到很好的量化。这样的动力学对于旨在描述金属粉末的热诱导点火的模型至关重要。另外,对于镁来说,老化的问题很重要,因为其氧化物氧化镁是多孔的。在这项工作中,通过对微米级球形粉末进行热分析测量,研究了氧气和蒸汽对镁的氧化作用。热流量热法和热重分析法分别用于量化低温和高温范围的反应速率。使用具有不同但重叠的粒度分布的球形粉末进行的实验来确定反应界面的位置。对于所有氧化条件,发现反应发生在金属和生长的氧化物层的界面处。因此,反应速度受到氧化剂向金属表面扩散的限制。使用热分析测量和不同的数据处理技术,可以对在干燥和潮湿氧化环境下的氧化动力学进行定量。在潮湿的环境中,低温下镁氧化的活化能接近60 kJ / mol。氧气中镁的氧化活化能为148 kJ / mol。为了在高温下氧化蒸汽中的镁,活化能从大约130 kJ / mol线性增加到360 kJ / mol,而氧化层的厚度增长到2.4μm。针对氧气和蒸汽中镁的氧化,开发了简化的扩散受限反应模型。该模型使人们能够预测在加热Mg颗粒时发生的提前点火反应以及在不同温度下暴露于湿气或氧气时Mg粉末老化的时间。

































 京公网安备 11010802027423号
京公网安备 11010802027423号