当前位置:
X-MOL 学术
›
Adv. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Amorphous Cobalt Boride (Co2B) as a Highly Efficient Nonprecious Catalyst for Electrochemical Water Splitting: Oxygen and Hydrogen Evolution
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2016-01-07 , DOI: 10.1002/aenm.201502313 Justus Masa 1 , Philipp Weide 2 , Daniel Peeters 3 , Ilya Sinev 2 , Wei Xia 2 , Zhenyu Sun 1 , Christoph Somsen 4 , Martin Muhler 2 , Wolfgang Schuhmann 1
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2016-01-07 , DOI: 10.1002/aenm.201502313 Justus Masa 1 , Philipp Weide 2 , Daniel Peeters 3 , Ilya Sinev 2 , Wei Xia 2 , Zhenyu Sun 1 , Christoph Somsen 4 , Martin Muhler 2 , Wolfgang Schuhmann 1
Affiliation
|
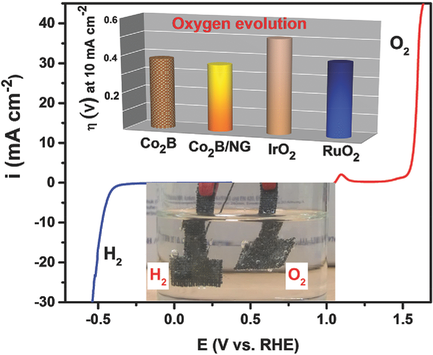
It is demonstrated that amorphous cobalt boride (Co2B) prepared by the chemical reduction of CoCl2 using NaBH4 is an exceptionally efficient electrocatalyst for the oxygen evolution reaction (OER) in alkaline electrolytes and is simultaneously active for catalyzing the hydrogen evolution reaction (HER). The catalyst achieves a current density of 10 mA cm−2 at 1.61 V on an inert support and at 1.59 V when impregnated with nitrogen‐doped graphene. Stable performance is maintained at 10 mA cm−2 for at least 60 h. The optimized catalyst, Co2B annealed at 500 °C (Co2B‐500) evolves oxygen more efficiently than RuO2 and IrO2, and its performance matches the best cobalt‐based catalysts reported to date. Co2B is irreversibly oxidized at OER conditions to form a CoOOH surface layer. The active form of the catalyst is therefore represented as CoOOH/Co2B. EXAFS observations indicate that boron induces lattice strain in the crystal structure of the metal, which potentially diminishes the thermodynamic and kinetic barrier of the hydroxylation reaction, formation of the OOH* intermediate, a key limiting step in the OER.
中文翻译:

非晶硼化钴(Co2B)作为高效非贵金属电化学水分解催化剂:氧气和氢气的释放
已证明通过使用NaBH 4对CoCl 2进行化学还原制备的无定形硼化钴(Co 2 B)是用于碱性电解液中氧释放反应(OER)的异常高效的电催化剂,同时具有催化氢释放反应的活性(她)。在惰性载体上,该催化剂在1.61 V时达到10 mA cm -2的电流密度,而在用氮掺杂石墨烯浸渍时则在1.59 V时达到10 mA cm -2。稳定的性能在10 mA cm -2的条件下保持至少60 h。经过优化的催化剂Co 2 B在500°C退火(Co 2 B-500)比RuO 2和IrO 2更有效地释放氧气,其性能与迄今为止报道的最好的钴基催化剂相匹配。Co 2 B在OER条件下不可逆地氧化形成CoOOH表面层。因此,催化剂的活性形式被表示为的CoOOH / Co的2 B. EXAFS观察结果表明,硼诱导金属,这潜在地削弱了羟基化反应的热力学和动力学障碍,OOH的形成的晶体结构的晶格应变*中间,这是OER中的关键限制步骤。
更新日期:2016-01-07
中文翻译:

非晶硼化钴(Co2B)作为高效非贵金属电化学水分解催化剂:氧气和氢气的释放
已证明通过使用NaBH 4对CoCl 2进行化学还原制备的无定形硼化钴(Co 2 B)是用于碱性电解液中氧释放反应(OER)的异常高效的电催化剂,同时具有催化氢释放反应的活性(她)。在惰性载体上,该催化剂在1.61 V时达到10 mA cm -2的电流密度,而在用氮掺杂石墨烯浸渍时则在1.59 V时达到10 mA cm -2。稳定的性能在10 mA cm -2的条件下保持至少60 h。经过优化的催化剂Co 2 B在500°C退火(Co 2 B-500)比RuO 2和IrO 2更有效地释放氧气,其性能与迄今为止报道的最好的钴基催化剂相匹配。Co 2 B在OER条件下不可逆地氧化形成CoOOH表面层。因此,催化剂的活性形式被表示为的CoOOH / Co的2 B. EXAFS观察结果表明,硼诱导金属,这潜在地削弱了羟基化反应的热力学和动力学障碍,OOH的形成的晶体结构的晶格应变*中间,这是OER中的关键限制步骤。





























 京公网安备 11010802027423号
京公网安备 11010802027423号