Synthesis ( IF 2.2 ) Pub Date : 2018-07-05 , DOI: 10.1055/s-0037-1609586 Tushar Chakraborty 1 , Dipendu Das 1 , Hina Khan 1
|
Published as part of the Special Topic Modern Radical Methods and their Strategic Applications in Synthesis
Abstract
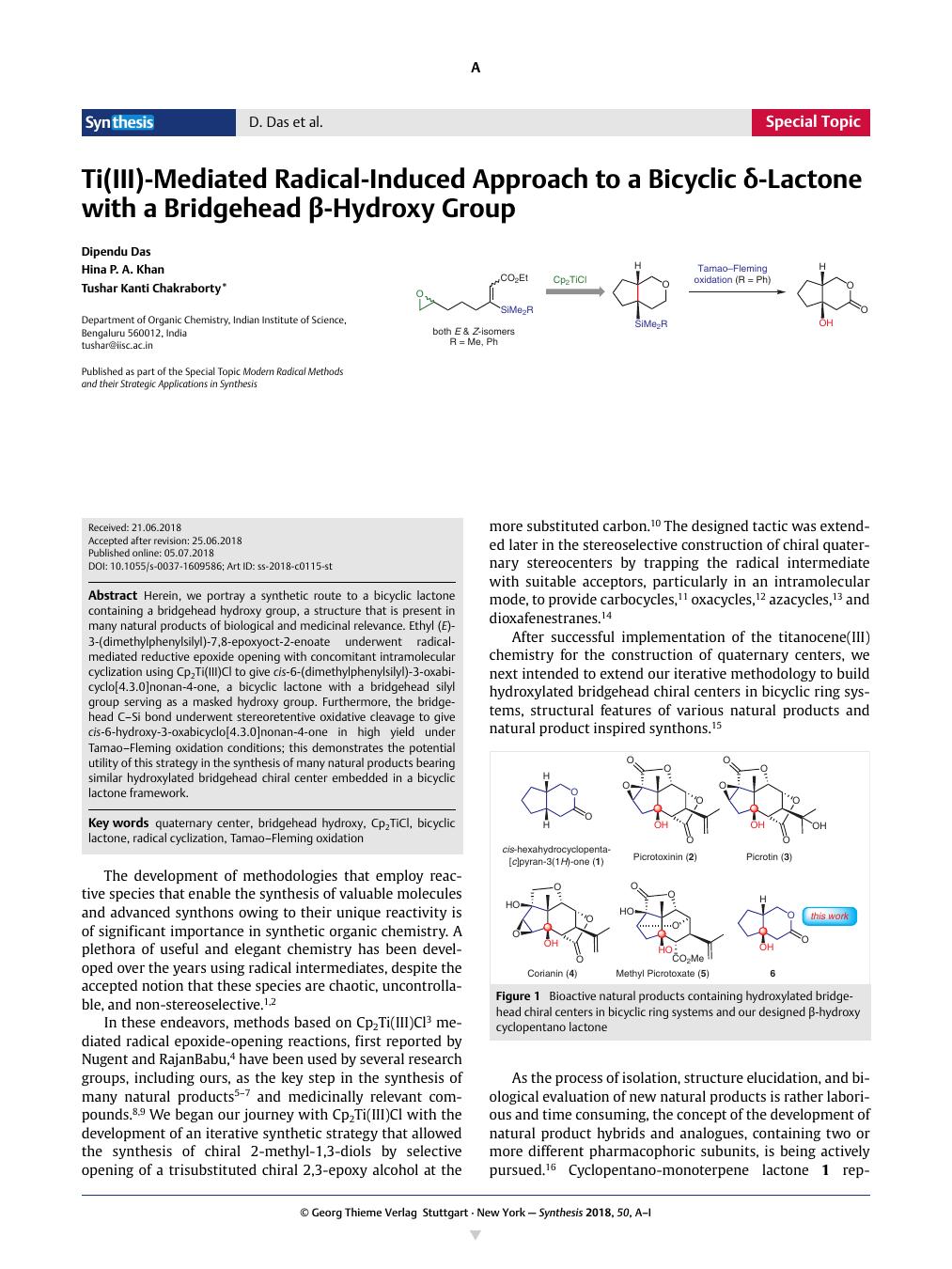
Herein, we portray a synthetic route to a bicyclic lactone containing a bridgehead hydroxy group, a structure that is present in many natural products of biological and medicinal relevance. Ethyl (E)-3-(dimethylphenylsilyl)-7,8-epoxyoct-2-enoate underwent radical-mediated reductive epoxide opening with concomitant intramolecular cyclization using Cp2Ti(III)Cl to give cis-6-(dimethylphenylsilyl)-3-oxabicyclo[4.3.0]nonan-4-one, a bicyclic lactone with a bridgehead silyl group serving as a masked hydroxy group. Furthermore, the bridgehead C–Si bond underwent stereoretentive oxidative cleavage to give cis-6-hydroxy-3-oxabicyclo[4.3.0]nonan-4-one in high yield under Tamao–Fleming oxidation conditions; this demonstrates the potential utility of this strategy in the synthesis of many natural products bearing similar hydroxylated bridgehead chiral center embedded in a bicyclic lactone framework.
Herein, we portray a synthetic route to a bicyclic lactone containing a bridgehead hydroxy group, a structure that is present in many natural products of biological and medicinal relevance. Ethyl (E)-3-(dimethylphenylsilyl)-7,8-epoxyoct-2-enoate underwent radical-mediated reductive epoxide opening with concomitant intramolecular cyclization using Cp2Ti(III)Cl to give cis-6-(dimethylphenylsilyl)-3-oxabicyclo[4.3.0]nonan-4-one, a bicyclic lactone with a bridgehead silyl group serving as a masked hydroxy group. Furthermore, the bridgehead C–Si bond underwent stereoretentive oxidative cleavage to give cis-6-hydroxy-3-oxabicyclo[4.3.0]nonan-4-one in high yield under Tamao–Fleming oxidation conditions; this demonstrates the potential utility of this strategy in the synthesis of many natural products bearing similar hydroxylated bridgehead chiral center embedded in a bicyclic lactone framework.
中文翻译:

Ti(III)介导的桥头β-羟基双环δ-内酯的自由基诱导方法
作为特别主题“现代自由基方法及其在合成中的战略应用”的一部分发布
抽象的
在本文中,我们描绘了一种合成方法,可合成含有桥头羟基的双环内酯,该结构存在于许多具有生物学和医学意义的天然产物中。(E)-3-(二甲基苯基甲硅烷基)-7,8-环氧辛-2-烯酸乙酯进行自由基介导的还原性环氧开放反应,并伴随分子内环化,使用Cp 2 Ti(III)Cl生成顺式-6-(二甲基苯基甲硅烷基)-3 -oxabicyclocyclo [4.3.0] nonan-4-one,一种双桥内酯,具有桥头甲硅烷基作为掩蔽的羟基。此外,桥头的C–Si键经历了立体保持性氧化裂解,形成顺式在Tamao-Fleming氧化条件下,高产-6-hydroxy-3-oxabicyclocyclo [4.3.0] nonan-4-one; 这证明了该策略在许多天然产物中的潜在效用,这些天然产物具有嵌入在双环内酯框架中的相似的羟基化桥头手性中心。
在本文中,我们描绘了一种合成方法,可合成含有桥头羟基的双环内酯,该结构存在于许多具有生物学和医学意义的天然产物中。(E)-3-(二甲基苯基甲硅烷基)-7,8-环氧辛-2-烯酸乙酯进行自由基介导的还原性环氧开放反应,并伴随分子内环化,使用Cp 2 Ti(III)Cl生成顺式-6-(二甲基苯基甲硅烷基)-3 -oxabicyclocyclo [4.3.0] nonan-4-one,一种双桥内酯,具有桥头甲硅烷基作为掩蔽的羟基。此外,桥头的C–Si键经历了立体保持性氧化裂解,形成顺式在Tamao-Fleming氧化条件下,高产-6-hydroxy-3-oxabicyclocyclo [4.3.0] nonan-4-one; 这证明了该策略在许多天然产物中的潜在效用,这些天然产物具有嵌入在双环内酯框架中的相似的羟基化桥头手性中心。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号