Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Targeting STING with covalent small-molecule inhibitors
Nature ( IF 50.5 ) Pub Date : 2018-07-01 , DOI: 10.1038/s41586-018-0287-8 Simone M Haag 1 , Muhammet F Gulen 1 , Luc Reymond 2 , Antoine Gibelin 2 , Laurence Abrami 1 , Alexiane Decout 1 , Michael Heymann 1 , F Gisou van der Goot 1 , Gerardo Turcatti 2 , Rayk Behrendt 3 , Andrea Ablasser 1
Nature ( IF 50.5 ) Pub Date : 2018-07-01 , DOI: 10.1038/s41586-018-0287-8 Simone M Haag 1 , Muhammet F Gulen 1 , Luc Reymond 2 , Antoine Gibelin 2 , Laurence Abrami 1 , Alexiane Decout 1 , Michael Heymann 1 , F Gisou van der Goot 1 , Gerardo Turcatti 2 , Rayk Behrendt 3 , Andrea Ablasser 1
Affiliation
|
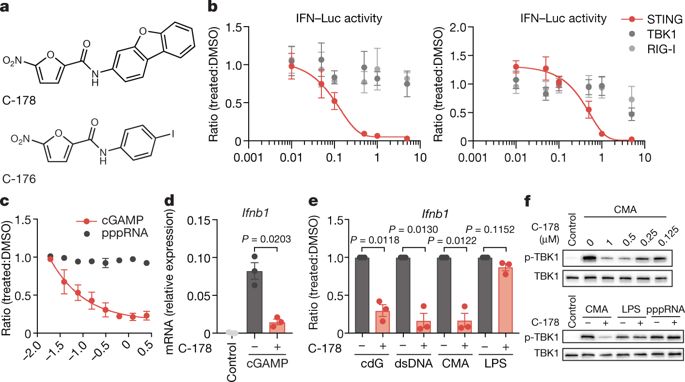
Aberrant activation of innate immune pathways is associated with a variety of diseases. Progress in understanding the molecular mechanisms of innate immune pathways has led to the promise of targeted therapeutic approaches, but the development of drugs that act specifically on molecules of interest remains challenging. Here we report the discovery and characterization of highly potent and selective small-molecule antagonists of the stimulator of interferon genes (STING) protein, which is a central signalling component of the intracellular DNA sensing pathway1,2. Mechanistically, the identified compounds covalently target the predicted transmembrane cysteine residue 91 and thereby block the activation-induced palmitoylation of STING. Using these inhibitors, we show that the palmitoylation of STING is essential for its assembly into multimeric complexes at the Golgi apparatus and, in turn, for the recruitment of downstream signalling factors. The identified compounds and their derivatives reduce STING-mediated inflammatory cytokine production in both human and mouse cells. Furthermore, we show that these small-molecule antagonists attenuate pathological features of autoinflammatory disease in mice. In summary, our work uncovers a mechanism by which STING can be inhibited pharmacologically and demonstrates the potential of therapies that target STING for the treatment of autoinflammatory disease.The discovery and characterization of small-molecule antagonists that inhibit the stimulator of interferon genes (STING) protein may help to develop therapies for the treatment of autoinflammatory disease.
中文翻译:

用共价小分子抑制剂靶向 STING
先天免疫通路的异常激活与多种疾病有关。对先天免疫通路分子机制的理解取得了进展,为靶向治疗方法带来了希望,但开发专门作用于目标分子的药物仍然具有挑战性。在这里,我们报告了干扰素基因 (STING) 蛋白刺激物的高效选择性小分子拮抗剂的发现和表征,STING 蛋白是细胞内 DNA 传感通路的核心信号传导成分 1,2。从机制上讲,已鉴定的化合物共价靶向预测的跨膜半胱氨酸残基 91,从而阻止激活诱导的 STING 棕榈酰化。使用这些抑制剂,我们表明,STING 的棕榈酰化对于其在高尔基体中组装成多聚体复合物是必不可少的,反过来,对于下游信号因子的募集也是必不可少的。已鉴定的化合物及其衍生物可减少人和小鼠细胞中 STING 介导的炎性细胞因子的产生。此外,我们表明这些小分子拮抗剂可减轻小鼠自身炎症性疾病的病理特征。总之,我们的工作揭示了一种可以通过药理学抑制 STING 的机制,并证明了靶向 STING 治疗自身炎症性疾病的潜力。 抑制干扰素基因刺激物 (STING) 的小分子拮抗剂的发现和表征蛋白质可能有助于开发治疗自身炎症性疾病的疗法。
更新日期:2018-07-01
中文翻译:

用共价小分子抑制剂靶向 STING
先天免疫通路的异常激活与多种疾病有关。对先天免疫通路分子机制的理解取得了进展,为靶向治疗方法带来了希望,但开发专门作用于目标分子的药物仍然具有挑战性。在这里,我们报告了干扰素基因 (STING) 蛋白刺激物的高效选择性小分子拮抗剂的发现和表征,STING 蛋白是细胞内 DNA 传感通路的核心信号传导成分 1,2。从机制上讲,已鉴定的化合物共价靶向预测的跨膜半胱氨酸残基 91,从而阻止激活诱导的 STING 棕榈酰化。使用这些抑制剂,我们表明,STING 的棕榈酰化对于其在高尔基体中组装成多聚体复合物是必不可少的,反过来,对于下游信号因子的募集也是必不可少的。已鉴定的化合物及其衍生物可减少人和小鼠细胞中 STING 介导的炎性细胞因子的产生。此外,我们表明这些小分子拮抗剂可减轻小鼠自身炎症性疾病的病理特征。总之,我们的工作揭示了一种可以通过药理学抑制 STING 的机制,并证明了靶向 STING 治疗自身炎症性疾病的潜力。 抑制干扰素基因刺激物 (STING) 的小分子拮抗剂的发现和表征蛋白质可能有助于开发治疗自身炎症性疾病的疗法。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号