Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
3-Dialkylamino-1,2,4-triazoles via ZnII-Catalyzed Acyl Hydrazide-Dialkylcyanamide Coupling.
ACS Omega ( IF 3.7 ) Pub Date : 2018-07-03 , DOI: 10.1021/acsomega.8b01047 Sevilya N Yunusova 1 , Dmitrii S Bolotin 1 , Vitalii V Suslonov 2 , Mikhail A Vovk 2 , Peter M Tolstoy 2 , Vadim Yu Kukushkin 1
ACS Omega ( IF 3.7 ) Pub Date : 2018-07-03 , DOI: 10.1021/acsomega.8b01047 Sevilya N Yunusova 1 , Dmitrii S Bolotin 1 , Vitalii V Suslonov 2 , Mikhail A Vovk 2 , Peter M Tolstoy 2 , Vadim Yu Kukushkin 1
Affiliation
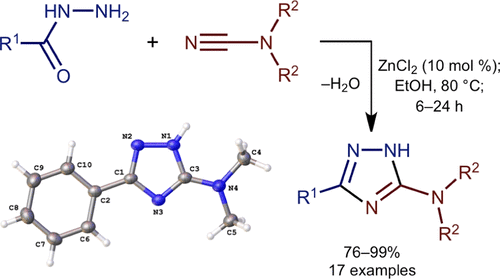
|
Zinc(II)-catalyzed (10 mol % ZnCl2) coupling of acyl hydrazides and dialkylcyanamides in ethanol leads to 3-dialkylamino-1,2,4-triazoles (76-99%; 17 examples). This reaction represents a novel, straightforward, and high-yielding approach to practically important 3-NR2-1,2,4-triazoles, which utilizes commercially available and/or easily generated substrates. Seventeen new 3-NR2-1,2,4-triazoles were characterized by HRESI+-MS and IR, 1H, and 13C{1H} NMR spectroscopies and five species additionally by single-crystal X-ray diffraction (XRD). The ZnII-catalyzed reaction proceeds via initial generation of the [Zn{RC(=O)NHNH2}3](ZnCl4) complexes (exemplified by isolation of the complex with R = Ph, 76%; characterized by HRESI+-MS, IR, CP-MAS TOSS 13C{1H} NMR, and XRD). Electronic effects of substituents at the acyl hydrazide moiety do not significantly affect the reaction rate and the yield of the target triazoles, whereas the steric hindrances reduce the reaction rate without affecting the yield of the heterocycles.
中文翻译:

3-二烷基氨基-1,2,4-三唑通过ZnII催化的酰肼-二烷基氰酰胺偶联。
乙醇中酰肼和二烷基氰酰胺的锌(II)催化(10 mol%ZnCl2)偶联反应生成3-二烷基氨基-1,2,4-三唑(76-99%; 17个实例)。该反应代表了对实用上重要的3-NR2-1,2,4-三唑的新颖,直接和高产率的方法,该方法利用了可商购的和/或易于产生的底物。通过HRESI + -MS和IR,1H和13C {1H} NMR光谱对17种新的3-NR2-1,2,4-三唑进行了表征,另外通过单晶X射线衍射(XRD)对了5种物质进行了表征。ZnII催化的反应通过[Zn {RC(= O)NHNH2} 3](ZnCl4)配合物的初始生成而进行(以R = Ph,76%的配合物分离为例;其特征为HRESI + -MS,IR, CP-MAS TOSS 13C {1H} NMR和XRD)。
更新日期:2018-07-03
中文翻译:

3-二烷基氨基-1,2,4-三唑通过ZnII催化的酰肼-二烷基氰酰胺偶联。
乙醇中酰肼和二烷基氰酰胺的锌(II)催化(10 mol%ZnCl2)偶联反应生成3-二烷基氨基-1,2,4-三唑(76-99%; 17个实例)。该反应代表了对实用上重要的3-NR2-1,2,4-三唑的新颖,直接和高产率的方法,该方法利用了可商购的和/或易于产生的底物。通过HRESI + -MS和IR,1H和13C {1H} NMR光谱对17种新的3-NR2-1,2,4-三唑进行了表征,另外通过单晶X射线衍射(XRD)对了5种物质进行了表征。ZnII催化的反应通过[Zn {RC(= O)NHNH2} 3](ZnCl4)配合物的初始生成而进行(以R = Ph,76%的配合物分离为例;其特征为HRESI + -MS,IR, CP-MAS TOSS 13C {1H} NMR和XRD)。















































 京公网安备 11010802027423号
京公网安备 11010802027423号