当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Recovering Valuable Metals from Spent Lithium Ion Battery via a Combination of Reduction Thermal Treatment and Facile Acid Leaching
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2018-07-03 00:00:00 , DOI: 10.1021/acssuschemeng.8b01805 Yang Yue 1 , Sun Wei 1 , Bu Yongjie 1 , Zhang Chenyang 1 , Song Shaole 1 , Hu Yuehua 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2018-07-03 00:00:00 , DOI: 10.1021/acssuschemeng.8b01805 Yang Yue 1 , Sun Wei 1 , Bu Yongjie 1 , Zhang Chenyang 1 , Song Shaole 1 , Hu Yuehua 1
Affiliation
|
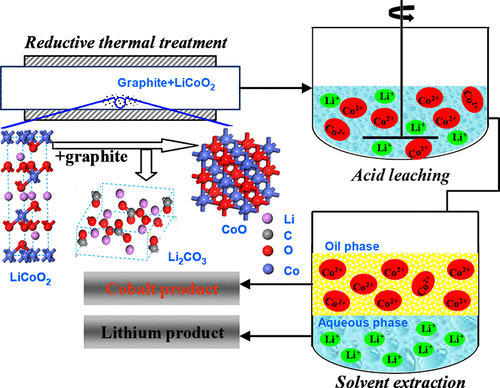
The traditional acid leaching process for releasing valuable metals from spent lithium-ion batteries (LIBs) is inefficient and inevitably consumes large amounts of reductants. In this study, a novel process, based on a reduction thermal treatment and reductant-free acid leaching, for recycling valuable metals from spent LIBs has been developed. First, a thermodynamics calculation was performed to judge whether the reducing reaction between LiCoO2 and graphite can occur or not. Then, reduction thermal treatment experiments were conducted. The process was tested by a thermogravimetry/differential thermal analysis method, and reaction products were measured by X-ray powder diffraction and X-ray photoelectron spectroscopy. The experimental results agree well with thermodynamics analysis, and the desired CoO and Li2CO3 were obtained under the optimum processing conditions of 600 °C, 120 min, and molar ratio of LiCoO2 to graphite of 2:1. Finally, almost 100% Li and Co were easily leached from the reaction product under the conditions of 2.25 M H2SO4, 80 °C, 30 min, and S/L = 100 g·L–1, and Co and Li in the leaching liquor were further separated with 35% PC88 at the ratio of aqueous to organic (A:O) equaling 0.5, 25 °C, and pH = 5.5. The proposed approach can not only make full utilization of waste anode graphite, but also benefit leaching valuable metals in the absence of reductant, which significantly improves the economy and recovery performance of recycling spent LIBs.
中文翻译:

还原热处理与酸浸法相结合,从废锂离子电池中回收有价值的金属
从废锂离子电池(LIB)中释放有价值的金属的传统酸浸工艺效率低下,不可避免地会消耗大量的还原剂。在这项研究中,已经开发了一种基于还原热处理和无还原剂酸浸的新方法,用于从废LIB中回收有价值的金属。首先,进行热力学计算以判断LiCoO 2和石墨之间的还原反应是否可能发生。然后,进行还原热处理实验。通过热重/差热分析方法测试该过程,并通过X射线粉末衍射和X射线光电子能谱测量反应产物。实验结果与热力学分析吻合良好,所需的CoO和Li在600℃,120分钟的最佳工艺条件下,LiCoO 2与石墨的摩尔比为2:1 ,获得了2个CO 3。最后,几乎100%的Li和Co容易从反应产物中的2.25 MH的条件下浸出2 SO 4,80℃,30分钟,S / L =100克·L -1,和Co与Li的用35%PC88进一步分离浸出液,水与有机物的比例(A:O)等于0.5、25°C,pH = 5.5。所提出的方法不仅可以充分利用阳极废石墨,而且还可以在没有还原剂的情况下浸出有价值的金属,从而显着提高回收废LIB的经济性和回收性能。
更新日期:2018-07-03
中文翻译:

还原热处理与酸浸法相结合,从废锂离子电池中回收有价值的金属
从废锂离子电池(LIB)中释放有价值的金属的传统酸浸工艺效率低下,不可避免地会消耗大量的还原剂。在这项研究中,已经开发了一种基于还原热处理和无还原剂酸浸的新方法,用于从废LIB中回收有价值的金属。首先,进行热力学计算以判断LiCoO 2和石墨之间的还原反应是否可能发生。然后,进行还原热处理实验。通过热重/差热分析方法测试该过程,并通过X射线粉末衍射和X射线光电子能谱测量反应产物。实验结果与热力学分析吻合良好,所需的CoO和Li在600℃,120分钟的最佳工艺条件下,LiCoO 2与石墨的摩尔比为2:1 ,获得了2个CO 3。最后,几乎100%的Li和Co容易从反应产物中的2.25 MH的条件下浸出2 SO 4,80℃,30分钟,S / L =100克·L -1,和Co与Li的用35%PC88进一步分离浸出液,水与有机物的比例(A:O)等于0.5、25°C,pH = 5.5。所提出的方法不仅可以充分利用阳极废石墨,而且还可以在没有还原剂的情况下浸出有价值的金属,从而显着提高回收废LIB的经济性和回收性能。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号