当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Revealing the Chemical Mechanism of NaO2 Decomposition by In Situ Raman Imaging
Chemistry of Materials ( IF 7.2 ) Pub Date : 2018-07-02 00:00:00 , DOI: 10.1021/acs.chemmater.8b01704 Hossein Yadegari 1 , Mohammad Norouzi Banis 1 , Xiaoting Lin 1 , Alicia Koo 1 , Ruying Li 1 , Xueliang Sun 1
Chemistry of Materials ( IF 7.2 ) Pub Date : 2018-07-02 00:00:00 , DOI: 10.1021/acs.chemmater.8b01704 Hossein Yadegari 1 , Mohammad Norouzi Banis 1 , Xiaoting Lin 1 , Alicia Koo 1 , Ruying Li 1 , Xueliang Sun 1
Affiliation
|
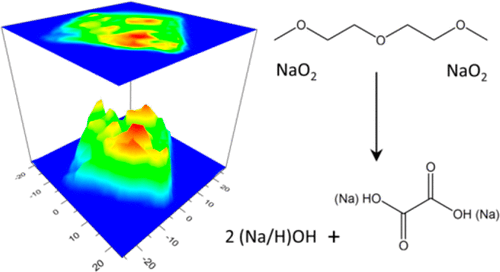
Sodium–oxygen (Na–O2) batteries exhibit a low charging overpotential owing to the reversible formation and decomposition of sodium superoxide (NaO2) on discharge and charge cycles. However, the cycling performance of the battery system is compromised by the side reactions occurring between the reactive NaO2 discharge product with the other components of the cell including the air electrode and the organic electrolyte. In the present study, we employ a Raman imaging technique to reveal the chemical mechanism behind the decomposition reaction of NaO2 in the presence of diglyme-based electrolyte. Our results illustrate the formation of oxalate-based side products resulting from prolonged exposure of NaO2 to the cell electrolyte. Moreover, we show that Na2O2·2H2O is not the thermodynamically favorable side product for decomposition of NaO2 and may only be formed under the high-energy beam used by the measuring probe. The findings of this study help to better understand the underlying chemical reaction mechanisms of Na–O2 cells.
中文翻译:

通过原位拉曼成像揭示NaO 2分解的化学机理
钠氧(Na-O 2)电池由于在放电和充电周期中可逆的超氧化钠(NaO 2)的形成和分解而显示出较低的充电过电势。然而,电池系统的循环性能受到反应性NaO 2放电产物与电池的其他部件(包括空气电极和有机电解质)之间发生的副反应的损害。在本研究中,我们采用拉曼成像技术来揭示在存在二甘醇二甲醚基电解质的情况下NaO 2分解反应背后的化学机理。我们的结果表明,长时间暴露于NaO 2会形成草酸盐基副产物到细胞电解质。而且,我们表明,Na 2 O 2 ·2H 2 O不是用于分解NaO 2的热力学上有利的副产物,而只能在测量探针使用的高能束下形成。这项研究的发现有助于更好地了解Na–O 2细胞的潜在化学反应机制。
更新日期:2018-07-02
中文翻译:

通过原位拉曼成像揭示NaO 2分解的化学机理
钠氧(Na-O 2)电池由于在放电和充电周期中可逆的超氧化钠(NaO 2)的形成和分解而显示出较低的充电过电势。然而,电池系统的循环性能受到反应性NaO 2放电产物与电池的其他部件(包括空气电极和有机电解质)之间发生的副反应的损害。在本研究中,我们采用拉曼成像技术来揭示在存在二甘醇二甲醚基电解质的情况下NaO 2分解反应背后的化学机理。我们的结果表明,长时间暴露于NaO 2会形成草酸盐基副产物到细胞电解质。而且,我们表明,Na 2 O 2 ·2H 2 O不是用于分解NaO 2的热力学上有利的副产物,而只能在测量探针使用的高能束下形成。这项研究的发现有助于更好地了解Na–O 2细胞的潜在化学反应机制。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号