当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cytochrome c Reduction by H2S Potentiates Sulfide Signaling.
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-07-18 , DOI: 10.1021/acschembio.8b00463
Victor Vitvitsky 1 , Jan Lj Miljkovic 2, 3 , Trever Bostelaar 1 , Bikash Adhikari 2, 3 , Pramod K Yadav 1 , Andrea K Steiger 4 , Roberta Torregrossa 5 , Michael D Pluth 4 , Matthew Whiteman 5 , Ruma Banerjee 1 , Milos R Filipovic 2, 3
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-07-18 , DOI: 10.1021/acschembio.8b00463
Victor Vitvitsky 1 , Jan Lj Miljkovic 2, 3 , Trever Bostelaar 1 , Bikash Adhikari 2, 3 , Pramod K Yadav 1 , Andrea K Steiger 4 , Roberta Torregrossa 5 , Michael D Pluth 4 , Matthew Whiteman 5 , Ruma Banerjee 1 , Milos R Filipovic 2, 3
Affiliation
|
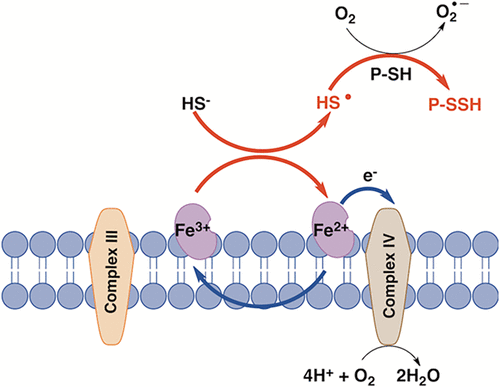
Hydrogen sulfide (H2S) is an endogenously produced gas that is toxic at high concentrations. It is eliminated by a dedicated mitochondrial sulfide oxidation pathway, which connects to the electron transfer chain at the level of complex III. Direct reduction of cytochrome c (Cyt C) by H2S has been reported previously but not characterized. In this study, we demonstrate that reduction of ferric Cyt C by H2S exhibits hysteretic behavior, which suggests the involvement of reactive sulfur species in the reduction process and is consistent with a reaction stoichiometry of 1.5 mol of Cyt C reduced/mol of H2S oxidized. H2S increases O2 consumption by human cells (HT29 and HepG2) treated with the complex III inhibitor antimycin A, which is consistent with the entry of sulfide-derived electrons at the level of complex IV. Cyt C-dependent H2S oxidation stimulated protein persulfidation in vitro, while silencing of Cyt C expression decreased mitochondrial protein persulfidation in a cell culture. Cyt C released during apoptosis was correlated with persulfidation of procaspase 9 and with loss of its activity. These results reveal a potential role for the electron transfer chain in general, and Cyt C in particular, for potentiating sulfide-based signaling.
中文翻译:

H2S 还原细胞色素 c 增强硫化物信号传导。
硫化氢 (H2S) 是一种内源产生的气体,高浓度时有毒。它通过专用的线粒体硫化物氧化途径消除,该途径连接到复合体 III 水平的电子传递链。先前已报道过 H2S 直接还原细胞色素 c (Cyt C),但尚未对其进行表征。在本研究中,我们证明 H2S 还原铁 Cyt C 表现出滞后行为,这表明还原过程中涉及活性硫物质,并且与 1.5 mol Cyt C 还原/mol H2S 氧化的反应化学计量一致。 H2S 增加了用复合物 III 抑制剂抗霉素 A 处理的人体细胞(HT29 和 HepG2)的 O2 消耗,这与硫化物衍生电子在复合物 IV 水平的进入一致。 Cyt C 依赖性 H2S 氧化刺激了体外蛋白质过硫化,而 Cyt C 表达沉默则减少了细胞培养物中线粒体蛋白质过硫化。细胞凋亡过程中释放的 Cyt C 与 procaspase 9 的过硫化及其活性丧失相关。这些结果揭示了电子传递链(特别是 Cyt C)在增强基于硫化物的信号传导方面的潜在作用。
更新日期:2018-07-02
中文翻译:

H2S 还原细胞色素 c 增强硫化物信号传导。
硫化氢 (H2S) 是一种内源产生的气体,高浓度时有毒。它通过专用的线粒体硫化物氧化途径消除,该途径连接到复合体 III 水平的电子传递链。先前已报道过 H2S 直接还原细胞色素 c (Cyt C),但尚未对其进行表征。在本研究中,我们证明 H2S 还原铁 Cyt C 表现出滞后行为,这表明还原过程中涉及活性硫物质,并且与 1.5 mol Cyt C 还原/mol H2S 氧化的反应化学计量一致。 H2S 增加了用复合物 III 抑制剂抗霉素 A 处理的人体细胞(HT29 和 HepG2)的 O2 消耗,这与硫化物衍生电子在复合物 IV 水平的进入一致。 Cyt C 依赖性 H2S 氧化刺激了体外蛋白质过硫化,而 Cyt C 表达沉默则减少了细胞培养物中线粒体蛋白质过硫化。细胞凋亡过程中释放的 Cyt C 与 procaspase 9 的过硫化及其活性丧失相关。这些结果揭示了电子传递链(特别是 Cyt C)在增强基于硫化物的信号传导方面的潜在作用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号