当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Rh(II)/Pd(0) Relay Catalysis: Synthesis of α-Quaternary Chiral β-Lactams through Enantioselective C–H Insertion/Diastereoselective Allylation of Diazoamides
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-06-29 00:00:00 , DOI: 10.1021/acscatal.8b01687 Liang-Zhu Huang 1, 2 , Zi Xuan 2 , Hyun Ji Jeon 2 , Zhen-Ting Du 1 , Ju Hyun Kim 3 , Sang-gi Lee 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-06-29 00:00:00 , DOI: 10.1021/acscatal.8b01687 Liang-Zhu Huang 1, 2 , Zi Xuan 2 , Hyun Ji Jeon 2 , Zhen-Ting Du 1 , Ju Hyun Kim 3 , Sang-gi Lee 2
Affiliation
|
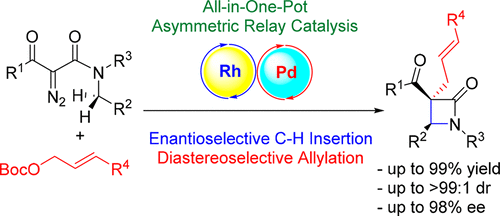
A straightforward route toward construction of α-quaternary chiral β-lactam moiety via Rh(II)/Pd(0)-catalyzed stereoselective relay catalytic reaction is reported. This asymmetric dual relay catalysis involves Rh(II)-catalyzed enantioselective intramoluecular C–H insertions of α-diazoamides, and sequential Pd(0)-catalyzed diastereoselective intermolecular allylic alkylation. Under mild reaction conditions, a broad range of α-quaternary allylated chiral β-lactams have been synthesized in high yields (up to 99%) with excellent stereoselectivities [up to diastereomeric ratio (dr) >99:1, up to 98% enantiomeric excess (ee)].
中文翻译:

不对称的Rh(II)/ Pd(0)中继催化:重氮酰胺的对映选择性C–H插入/非对映选择性烯丙基化反应合成α-四元手性β-内酰胺
据报道,通过Rh(II)/ Pd(0)催化的立体选择性中继催化反应,可直接构建α-季手性β-内酰胺部分。这种不对称的双重中继催化涉及Rh(II)催化的α-重氮酰胺的对映选择性分子内C–H插入,以及顺序的Pd(0)催化的非对映选择性分子间烯丙基烷基化。在温和的反应条件下,已以高收率(高达99%)和优异的立体选择性合成了多种α-季烯丙基手性β-内酰胺[对映异构体比率(dr)> 99:1,对映体高达98%超额(ee)]。
更新日期:2018-06-29
中文翻译:

不对称的Rh(II)/ Pd(0)中继催化:重氮酰胺的对映选择性C–H插入/非对映选择性烯丙基化反应合成α-四元手性β-内酰胺
据报道,通过Rh(II)/ Pd(0)催化的立体选择性中继催化反应,可直接构建α-季手性β-内酰胺部分。这种不对称的双重中继催化涉及Rh(II)催化的α-重氮酰胺的对映选择性分子内C–H插入,以及顺序的Pd(0)催化的非对映选择性分子间烯丙基烷基化。在温和的反应条件下,已以高收率(高达99%)和优异的立体选择性合成了多种α-季烯丙基手性β-内酰胺[对映异构体比率(dr)> 99:1,对映体高达98%超额(ee)]。































 京公网安备 11010802027423号
京公网安备 11010802027423号