当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Improved Doxorubicin Encapsulation and Pharmacokinetics of Ferritin–Fusion Protein Nanocarriers Bearing Proline, Serine, and Alanine Elements
Biomacromolecules ( IF 5.5 ) Pub Date : 2015-12-30 00:00:00 , DOI: 10.1021/acs.biomac.5b01446 Elisabetta Falvo 1, 2 , Elisa Tremante 3 , Alessandro Arcovito 4 , Massimiliano Papi 5 , Nadav Elad 6 , Alberto Boffi 1, 2, 7 , Veronica Morea 1 , Giamaica Conti 8 , Giuseppe Toffoli 9 , Giulio Fracasso 10 , Patrizio Giacomini 3 , Pierpaolo Ceci 1
Biomacromolecules ( IF 5.5 ) Pub Date : 2015-12-30 00:00:00 , DOI: 10.1021/acs.biomac.5b01446 Elisabetta Falvo 1, 2 , Elisa Tremante 3 , Alessandro Arcovito 4 , Massimiliano Papi 5 , Nadav Elad 6 , Alberto Boffi 1, 2, 7 , Veronica Morea 1 , Giamaica Conti 8 , Giuseppe Toffoli 9 , Giulio Fracasso 10 , Patrizio Giacomini 3 , Pierpaolo Ceci 1
Affiliation
|
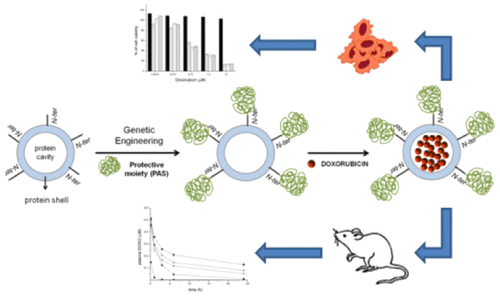
A novel human ferritin-based nanocarrier, composed of 24 modified monomers able to auto-assemble into a modified protein cage, was produced and used as selective carrier of anti-tumor payloads. Each modified monomer derives from the genetic fusion of two distinct modules, namely the heavy chain of human ferritin (HFt) and a stabilizing/protective PAS polypeptide sequence rich in proline (P), serine (S), and alanine (A) residues. Two genetically fused protein constructs containing PAS polymers with 40- and 75-residue lengths, respectively, were compared. They were produced and purified as recombinant proteins in Escherichia coli at high yields. Both preparations were highly soluble and stable in vitro as well as in mouse plasma. Size-exclusion chromatography, dynamic light scattering, and transmission electron microscopy results indicated that PASylated ferritins are fully assembled and highly monodispersed. In addition, yields and stability of encapsulated doxorubicin were significantly better for both HFt-PAS proteins than for wild-type HFt. Importantly, PAS sequences considerably prolonged the half-life of HFt in the mouse bloodstream. Finally, our doxorubicin-loaded nanocages preserved the pharmacological activity of the drug. Taken together, these results indicate that both of the developed HFt-PAS fusion proteins are promising nanocarriers for future applications in cancer therapy.
中文翻译:

改善了带有脯氨酸,丝氨酸和丙氨酸元素的铁蛋白融合蛋白纳米载体对阿霉素的包封和药代动力学
一种新型的基于人铁蛋白的纳米载体由24种能够自动组装到修饰的蛋白质笼中的修饰单体组成,并被用作抗肿瘤有效载荷的选择性载体。每个修饰的单体均来自两个不同模块的基因融合,即人铁蛋白的重链(HFt)和富含脯氨酸(P),丝氨酸(S)和丙氨酸(A)残基的稳定/保护性PAS多肽序列。比较了两种分别含有长度为40和75个残基的PAS聚合物的基因融合蛋白构建体。它们在大肠杆菌中以重组蛋白的高产量生产和纯化。两种制剂在体外均具有高度溶解性和稳定性以及小鼠血浆中。尺寸排阻色谱,动态光散射和透射电子显微镜结果表明,PASy化的铁蛋白完全组装且高度单分散。此外,两种HFt-PAS蛋白的包封阿霉素的产量和稳定性均明显优于野生型HFt。重要的是,PAS序列可大大延长HFt在小鼠血流中的半衰期。最后,我们装载阿霉素的纳米笼保留了该药的药理活性。综上所述,这些结果表明,已开发的两种HFt-PAS融合蛋白都是有前途的纳米载体,可用于癌症治疗的未来应用。
更新日期:2015-12-30
中文翻译:

改善了带有脯氨酸,丝氨酸和丙氨酸元素的铁蛋白融合蛋白纳米载体对阿霉素的包封和药代动力学
一种新型的基于人铁蛋白的纳米载体由24种能够自动组装到修饰的蛋白质笼中的修饰单体组成,并被用作抗肿瘤有效载荷的选择性载体。每个修饰的单体均来自两个不同模块的基因融合,即人铁蛋白的重链(HFt)和富含脯氨酸(P),丝氨酸(S)和丙氨酸(A)残基的稳定/保护性PAS多肽序列。比较了两种分别含有长度为40和75个残基的PAS聚合物的基因融合蛋白构建体。它们在大肠杆菌中以重组蛋白的高产量生产和纯化。两种制剂在体外均具有高度溶解性和稳定性以及小鼠血浆中。尺寸排阻色谱,动态光散射和透射电子显微镜结果表明,PASy化的铁蛋白完全组装且高度单分散。此外,两种HFt-PAS蛋白的包封阿霉素的产量和稳定性均明显优于野生型HFt。重要的是,PAS序列可大大延长HFt在小鼠血流中的半衰期。最后,我们装载阿霉素的纳米笼保留了该药的药理活性。综上所述,这些结果表明,已开发的两种HFt-PAS融合蛋白都是有前途的纳米载体,可用于癌症治疗的未来应用。































 京公网安备 11010802027423号
京公网安备 11010802027423号