当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Self-supported hierarchical CuOx@Co3O4 heterostructures as efficient bifunctional electrocatalysts for water splitting†
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2018-06-29 00:00:00 , DOI: 10.1039/c8ta03120g Qianqian Zhou 1, 2, 3, 4, 5 , Ting-Ting Li 1, 2, 3, 4, 5 , Jinjie Qian 5, 6, 7, 8 , Yue Hu 5, 6, 7, 8 , Fenya Guo 1, 2, 3, 4, 5 , Yue-Qing Zheng 1, 2, 3, 4, 5
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2018-06-29 00:00:00 , DOI: 10.1039/c8ta03120g Qianqian Zhou 1, 2, 3, 4, 5 , Ting-Ting Li 1, 2, 3, 4, 5 , Jinjie Qian 5, 6, 7, 8 , Yue Hu 5, 6, 7, 8 , Fenya Guo 1, 2, 3, 4, 5 , Yue-Qing Zheng 1, 2, 3, 4, 5
Affiliation
|
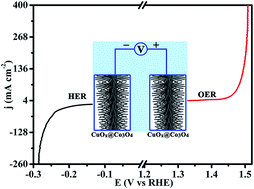
The preparation of low-cost and efficient bifunctional catalysts towards water splitting is essential for the production of clean H2 energy. Herein, a hierarchical Co3O4-decorated CuO–Cu2O nanorod core–shell structure was in situ grown on a Cu foam (denoted as CuOx@Co3O4 NRs/CF) and investigated as a bifunctional catalyst for both the oxygen evolution reaction (OER) and hydrogen evolution reaction (HER) in alkaline solution. Benefiting from its large electrochemical surface area and the synergetic effects between the CuOx core and Co3O4 shell, CuOx@Co3O4 NRs/CF exhibited considerable catalytic activity, which resulted in a small overpotential of 240 mV for the OER and 242 mV for the HER at a current density of 50 mA cm−2, along with low Tafel slopes of 46 and 61 mV dec−1, respectively. Remarkably, CuOx@Co3O4 NRs/CF could continuously produce O2 or H2 for at least 24 h with negligible decline in catalytic activity, and it gave rise to high faradaic efficiencies of 99.7% and 96.4% for the OER and HER, respectively. The electrochemical performance of CuOx@Co3O4 NRs/CF was dramatically improved as compared to those of its CuOx NRs/CF counterpart and also most reported Cu-based water splitting electrocatalysts.
中文翻译:

自支撑的分级CuO x @Co 3 O 4杂结构作为高效的双功能水分解双功能电催化剂†
制备低成本,高效的双官能度催化剂以进行水分解对于生产清洁的H 2能源至关重要。在这里,分层的Co 3 O 4修饰的CuO–Cu 2 O纳米棒核壳结构在Cu泡沫(表示为CuO x @Co 3 O 4 NRs / CF)上原位生长,并被研究为用于两者的双功能催化剂碱性溶液中的氧释放反应(OER)和氢释放反应(HER)。受益于其较大的电化学表面积以及CuO x核与Co 3 O 4壳CuO之间的协同作用x @Co 3 O 4 NRs / CF表现出相当大的催化活性,导致在50 mA cm -2的电流密度下,OER的小过电位为240 mV,HER的小过电位为242 mV,同时塔菲尔斜率低,为46和分别为61 mV dec -1。值得注意的是,CuO x @Co 3 O 4 NRs / CF可以连续产生O 2或H 2至少持续24 h,而催化活性却可以忽略不计,从而导致法拉第效率高达99.7%和96.4%。她,分别。CuO x @Co 3 O 4的电化学性能NRs / CF与CuO x NRs / CF对应物以及大多数报道的基于Cu的水分解电催化剂相比,均得到了显着改善。
更新日期:2018-06-29
中文翻译:

自支撑的分级CuO x @Co 3 O 4杂结构作为高效的双功能水分解双功能电催化剂†
制备低成本,高效的双官能度催化剂以进行水分解对于生产清洁的H 2能源至关重要。在这里,分层的Co 3 O 4修饰的CuO–Cu 2 O纳米棒核壳结构在Cu泡沫(表示为CuO x @Co 3 O 4 NRs / CF)上原位生长,并被研究为用于两者的双功能催化剂碱性溶液中的氧释放反应(OER)和氢释放反应(HER)。受益于其较大的电化学表面积以及CuO x核与Co 3 O 4壳CuO之间的协同作用x @Co 3 O 4 NRs / CF表现出相当大的催化活性,导致在50 mA cm -2的电流密度下,OER的小过电位为240 mV,HER的小过电位为242 mV,同时塔菲尔斜率低,为46和分别为61 mV dec -1。值得注意的是,CuO x @Co 3 O 4 NRs / CF可以连续产生O 2或H 2至少持续24 h,而催化活性却可以忽略不计,从而导致法拉第效率高达99.7%和96.4%。她,分别。CuO x @Co 3 O 4的电化学性能NRs / CF与CuO x NRs / CF对应物以及大多数报道的基于Cu的水分解电催化剂相比,均得到了显着改善。






























 京公网安备 11010802027423号
京公网安备 11010802027423号