Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
OTULIN limits cell death and inflammation by deubiquitinating LUBAC
Nature ( IF 50.5 ) Pub Date : 2018-06-27 , DOI: 10.1038/s41586-018-0256-2
Klaus Heger , Katherine E. Wickliffe , Ada Ndoja , Juan Zhang , Aditya Murthy , Debra L. Dugger , Allie Maltzman , Felipe de Sousa e Melo , Jeffrey Hung , Yi Zeng , Erik Verschueren , Donald S. Kirkpatrick , Domagoj Vucic , Wyne P. Lee , Merone Roose-Girma , Robert J. Newman , Søren Warming , Yi-Chun Hsiao , László G. Kőműves , Joshua D. Webster , Kim Newton , Vishva M. Dixit
Nature ( IF 50.5 ) Pub Date : 2018-06-27 , DOI: 10.1038/s41586-018-0256-2
Klaus Heger , Katherine E. Wickliffe , Ada Ndoja , Juan Zhang , Aditya Murthy , Debra L. Dugger , Allie Maltzman , Felipe de Sousa e Melo , Jeffrey Hung , Yi Zeng , Erik Verschueren , Donald S. Kirkpatrick , Domagoj Vucic , Wyne P. Lee , Merone Roose-Girma , Robert J. Newman , Søren Warming , Yi-Chun Hsiao , László G. Kőműves , Joshua D. Webster , Kim Newton , Vishva M. Dixit
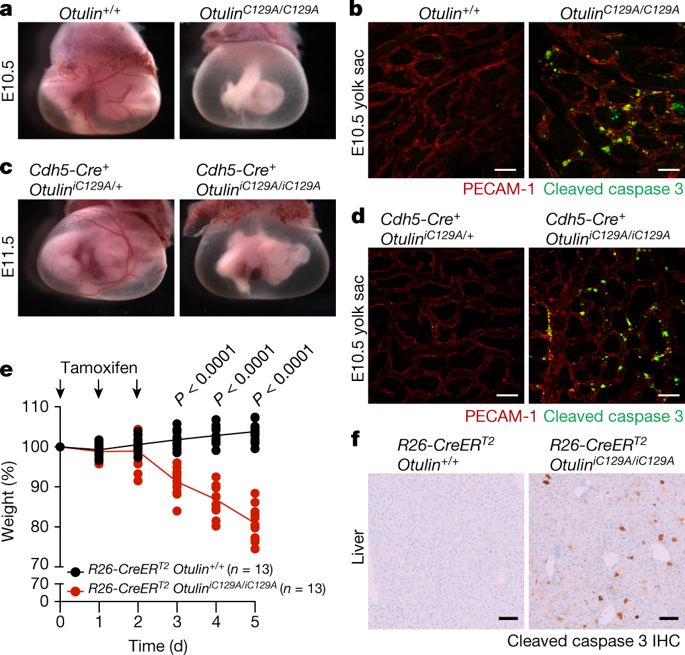
|
OTULIN (OTU deubiquitinase with linear linkage specificity) removes linear polyubiquitin from proteins that have been modified by LUBAC (linear ubiquitin chain assembly complex) and is critical for preventing auto-inflammatory disease1,2 and embryonic lethality during mouse development3. Here we show that OTULIN promotes rather than counteracts LUBAC activity by preventing its auto-ubiquitination with linear polyubiquitin. Thus, knock-in mice that express catalytically inactive OTULIN, either constitutively or selectively in endothelial cells, resembled LUBAC-deficient mice4 and died midgestation as a result of cell death mediated by TNFR1 (tumour necrosis factor receptor 1) and the kinase activity of RIPK1 (receptor-interacting protein kinase 1). Inactivation of OTULIN in adult mice also caused pro-inflammatory cell death. Accordingly, embryonic lethality and adult auto-inflammation were prevented by the combined loss of cell death mediators: caspase 8 for apoptosis and RIPK3 for necroptosis. Unexpectedly, OTULIN mutant mice that lacked caspase 8 and RIPK3 died in the perinatal period, exhibiting enhanced production of type I interferon that was dependent on RIPK1. Collectively, our results indicate that OTULIN and LUBAC function in a linear pathway, and highlight a previously unrecognized interaction between linear ubiquitination, regulators of cell death, and induction of type I interferon.OTULIN, which removes ubiquitin chains deposited by LUBAC, promotes LUBAC activity by preventing its auto-ubiquitination, thereby supporting normal mouse embryo development and preventing pro-inflammatory cell death in adult mice.
中文翻译:

OTULIN 通过去泛素化 LUBAC 限制细胞死亡和炎症
OTULIN(具有线性连接特异性的 OTU 脱泛素酶)从已被 LUBAC(线性泛素链组装复合物)修饰的蛋白质中去除线性多聚泛素,并且对于预防小鼠发育过程中的自身炎症疾病 1,2 和胚胎致死至关重要 3。在这里,我们表明 OTULIN 通过阻止其与线性多聚泛素的自动泛素化来促进而不是抵消 LUBAC 活性。因此,在内皮细胞中组成性或选择性表达催化失活 OTULIN 的敲入小鼠类似于 LUBAC 缺陷小鼠 4,并且由于 TNFR1(肿瘤坏死因子受体 1)和 RIPK1 的激酶活性介导的细胞死亡而在妊娠中期死亡(受体相互作用蛋白激酶 1)。成年小鼠中 OTULIN 的失活也会导致促炎细胞死亡。因此,细胞死亡介质(用于细胞凋亡的半胱天冬酶 8 和用于坏死性凋亡的 RIPK3)的联合损失阻止了胚胎致死率和成人自身炎症。出乎意料的是,缺乏 caspase 8 和 RIPK3 的 OTULIN 突变小鼠在围产期死亡,表现出依赖 RIPK1 的 I 型干扰素的产生增加。总的来说,我们的结果表明 OTULIN 和 LUBAC 在线性途径中起作用,并突出了线性泛素化、细胞死亡调节剂和 I 型干扰素诱导之间以前未被认识的相互作用。 OTULIN 去除了 LUBAC 沉积的泛素链,促进了 LUBAC 活性通过阻止其自身泛素化,从而支持正常的小鼠胚胎发育并防止成年小鼠的促炎细胞死亡。
更新日期:2018-06-27
中文翻译:

OTULIN 通过去泛素化 LUBAC 限制细胞死亡和炎症
OTULIN(具有线性连接特异性的 OTU 脱泛素酶)从已被 LUBAC(线性泛素链组装复合物)修饰的蛋白质中去除线性多聚泛素,并且对于预防小鼠发育过程中的自身炎症疾病 1,2 和胚胎致死至关重要 3。在这里,我们表明 OTULIN 通过阻止其与线性多聚泛素的自动泛素化来促进而不是抵消 LUBAC 活性。因此,在内皮细胞中组成性或选择性表达催化失活 OTULIN 的敲入小鼠类似于 LUBAC 缺陷小鼠 4,并且由于 TNFR1(肿瘤坏死因子受体 1)和 RIPK1 的激酶活性介导的细胞死亡而在妊娠中期死亡(受体相互作用蛋白激酶 1)。成年小鼠中 OTULIN 的失活也会导致促炎细胞死亡。因此,细胞死亡介质(用于细胞凋亡的半胱天冬酶 8 和用于坏死性凋亡的 RIPK3)的联合损失阻止了胚胎致死率和成人自身炎症。出乎意料的是,缺乏 caspase 8 和 RIPK3 的 OTULIN 突变小鼠在围产期死亡,表现出依赖 RIPK1 的 I 型干扰素的产生增加。总的来说,我们的结果表明 OTULIN 和 LUBAC 在线性途径中起作用,并突出了线性泛素化、细胞死亡调节剂和 I 型干扰素诱导之间以前未被认识的相互作用。 OTULIN 去除了 LUBAC 沉积的泛素链,促进了 LUBAC 活性通过阻止其自身泛素化,从而支持正常的小鼠胚胎发育并防止成年小鼠的促炎细胞死亡。

































 京公网安备 11010802027423号
京公网安备 11010802027423号