当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
4′‐Phenyl‐2,2′:6′,2′′‐terpyridine Derivatives Containing 1‐Substituted‐2,3‐Triazole Ring: Synthesis, Characterization and Anticancer Activity
ChemistrySelect ( IF 1.9 ) Pub Date : 2018-06-27 , DOI: 10.1002/slct.201801204 Dawid Zych 1 , Aneta Slodek 1 , Stanisław Krompiec 1 , Katarzyna Malarz 2 , Anna Mrozek-Wilczkiewicz 2 , Robert Musiol 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2018-06-27 , DOI: 10.1002/slct.201801204 Dawid Zych 1 , Aneta Slodek 1 , Stanisław Krompiec 1 , Katarzyna Malarz 2 , Anna Mrozek-Wilczkiewicz 2 , Robert Musiol 1
Affiliation
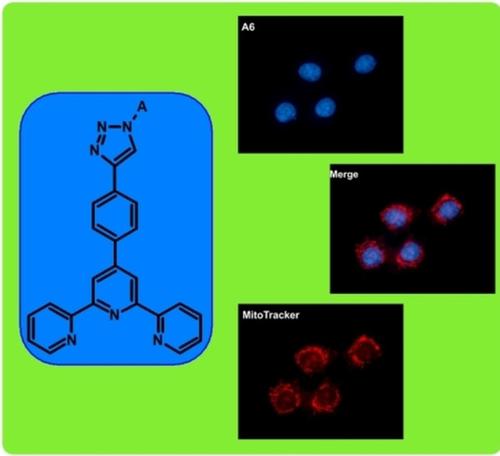
|
Terpyridine moiety is known for chelating and interesting physicochemical properties enabling some applications. Its biological activity, particularly anticancer potency is not well studied. Herein we present six 4′‐(1‐substituted‐2,3‐triazol‐4‐yl)phenyl‐2,2′:6′,2′′‐terpyridine derivatives with glycidyl, cyclohexylmethyl, benzyl, 4‐tert‐butylbenzyl, pentafluorobenzyl, and 2,6‐dichlorobenzyl groups which were obtained via Cu(I)‐catalysed 1,3‐dipolar cycloaddition reaction. The photophysical such as optical (absorption and photoluminescence spectra, quantum yields and lifetimes) and thermal (by using differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA)) properties were examined. In addition, theoretical studies (DFT and TD‐DFT) were performed to provide a deeper understanding of the experimental results. In vitro studies on the obtained novel structures were conducted with selected series of cell lines to check their bioactivity and toxicity. The most active compound reached a nanomolar level with good selectivity index better than doxorubicin. Mechanism of action for these terpyridines was also investigated which suggest intercalation of DNA as a trigger of cell death.
中文翻译:

含有1-取代-2,3-三唑环的4'-苯基-2-2,2':6',2''-吡啶吡啶衍生物:合成,表征和抗癌活性
特吡啶吡啶部分因其螯合和令人感兴趣的理化性质而闻名,可实现某些应用。其生物学活性,特别是抗癌能力尚未得到很好的研究。本文介绍了6种4'-(1-取代-2,3-三唑-4-基)苯基-2,2':6',2''-吡啶吡啶衍生物,其中有缩水甘油基,环己基甲基,苄基,4-叔丁基通过Cu(I)催化的1,3-偶极环加成反应获得的-丁基苄基,五氟苄基和2,6-二氯苄基。检查了光物理性质,如光学性质(吸收和光致发光光谱,量子产率和寿命)和热性质(通过使用差示扫描量热法(DSC)和热重分析(TGA))。此外,进行了理论研究(DFT和TD-DFT),以提供对实验结果的更深入的了解。体外使用选定的系列细胞系对获得的新型结构进行了研究,以检查其生物活性和毒性。活性最高的化合物达到纳摩尔水平,具有比阿霉素更好的选择性指数。还研究了这些三联吡啶的作用机理,表明插入DNA可引发细胞死亡。
更新日期:2018-06-27
中文翻译:

含有1-取代-2,3-三唑环的4'-苯基-2-2,2':6',2''-吡啶吡啶衍生物:合成,表征和抗癌活性
特吡啶吡啶部分因其螯合和令人感兴趣的理化性质而闻名,可实现某些应用。其生物学活性,特别是抗癌能力尚未得到很好的研究。本文介绍了6种4'-(1-取代-2,3-三唑-4-基)苯基-2,2':6',2''-吡啶吡啶衍生物,其中有缩水甘油基,环己基甲基,苄基,4-叔丁基通过Cu(I)催化的1,3-偶极环加成反应获得的-丁基苄基,五氟苄基和2,6-二氯苄基。检查了光物理性质,如光学性质(吸收和光致发光光谱,量子产率和寿命)和热性质(通过使用差示扫描量热法(DSC)和热重分析(TGA))。此外,进行了理论研究(DFT和TD-DFT),以提供对实验结果的更深入的了解。体外使用选定的系列细胞系对获得的新型结构进行了研究,以检查其生物活性和毒性。活性最高的化合物达到纳摩尔水平,具有比阿霉素更好的选择性指数。还研究了这些三联吡啶的作用机理,表明插入DNA可引发细胞死亡。







































 京公网安备 11010802027423号
京公网安备 11010802027423号