当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The “missing” bicarbonate in CO2 chemisorption reactions on solid amine sorbents
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-06-27 , DOI: 10.1021/jacs.8b04520 Chia-Hsin Chen 1 , Daphna Shimon 1 , Jason J Lee 2 , Frederic Mentink-Vigier 3 , Ivan Hung 3 , Carsten Sievers 2 , Christopher W Jones 2 , Sophia E Hayes 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-06-27 , DOI: 10.1021/jacs.8b04520 Chia-Hsin Chen 1 , Daphna Shimon 1 , Jason J Lee 2 , Frederic Mentink-Vigier 3 , Ivan Hung 3 , Carsten Sievers 2 , Christopher W Jones 2 , Sophia E Hayes 1
Affiliation
|
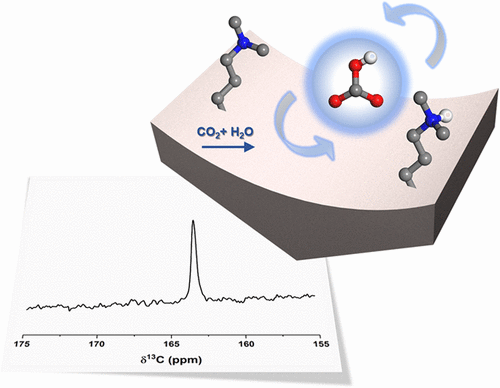
We have identified a hydrated bicarbonate formed by chemisorption of 13CO2 on both dimethylaminopropylsilane (DMAPS) and aminopropylsilane (APS) pendant molecules grafted on SBA-15 mesoporous silica. The most commonly used sequence in solid-state NMR, 13C CPMAS, failed to detect bicarbonate in these solid amine sorbent samples; here, we have employed a Bloch decay ("pulse-acquire") sequence (with 1H decoupling) to detect such species. The water that is present contributes to the dynamic motion of the bicarbonate product, thwarting CPMAS but enabling direct 13C detection by shortening the spin-lattice relaxation time. Since solid-state NMR plays a major role in characterizing chemisorption reactions, these new insights that allow for the routine detection of previously elusive bicarbonate species (which are also challenging to observe in IR spectroscopy) represent an important advance. We note that employing this straightforward NMR technique can reveal the presence of bicarbonate that has often otherwise been overlooked, as demonstrated in APS, that has been thought to only contain adsorbed CO2 as carbamate and carbamic acid species. As in other systems (e.g., proteins), dynamic species that sample multiple environments tend to broaden as their motion is frozen out. Here, we show two distinct bicarbonate species upon freezing, and coupling to different protons is shown through preliminary 13C-1H HETCOR measurements. This work demonstrates that bicarbonates have likely been formed in the presence of water but have gone unobserved by NMR due to the nature of the experiments most routinely employed, a perspective that will transform the way the sorption community will view CO2 capture by amines.
中文翻译:

固体胺吸附剂 CO2 化学吸附反应中“缺失”的碳酸氢盐
我们已经鉴定出通过 13CO2 化学吸附在接枝在 SBA-15 介孔二氧化硅上的二甲基氨基丙基硅烷 (DMAPS) 和氨基丙基硅烷 (APS) 侧分子上形成的水合碳酸氢盐。固态 NMR 中最常用的序列 13C CPMAS 未能检测到这些固体胺吸附剂样品中的碳酸氢盐;在这里,我们采用了布洛赫衰变(“脉冲获取”)序列(具有 1H 解耦)来检测此类物质。存在的水有助于碳酸氢盐产物的动态运动,阻碍 CPMAS,但可以通过缩短自旋晶格弛豫时间来实现直接 13C 检测。由于固态核磁共振在表征化学吸附反应方面发挥着重要作用,因此这些新见解可以对以前难以捉摸的碳酸氢盐物质(在红外光谱中观察到也具有挑战性)进行常规检测,这代表了一项重要的进步。我们注意到,采用这种简单的 NMR 技术可以揭示碳酸氢盐的存在,而碳酸氢盐通常被忽视,如 APS 所示,人们认为碳酸氢盐仅含有以氨基甲酸盐和氨基甲酸形式吸附的二氧化碳。与其他系统(例如蛋白质)一样,对多种环境进行采样的动态物种往往会随着其运动被冻结而扩大。在这里,我们在冷冻时显示了两种不同的碳酸氢盐物种,并通过初步 13C-1H HETCOR 测量显示了与不同质子的耦合。这项工作表明,碳酸氢盐很可能是在有水的情况下形成的,但由于最常用的实验的性质,核磁共振没有观察到碳酸氢盐,这一观点将改变吸附界看待胺捕获二氧化碳的方式。
更新日期:2018-06-27
中文翻译:

固体胺吸附剂 CO2 化学吸附反应中“缺失”的碳酸氢盐
我们已经鉴定出通过 13CO2 化学吸附在接枝在 SBA-15 介孔二氧化硅上的二甲基氨基丙基硅烷 (DMAPS) 和氨基丙基硅烷 (APS) 侧分子上形成的水合碳酸氢盐。固态 NMR 中最常用的序列 13C CPMAS 未能检测到这些固体胺吸附剂样品中的碳酸氢盐;在这里,我们采用了布洛赫衰变(“脉冲获取”)序列(具有 1H 解耦)来检测此类物质。存在的水有助于碳酸氢盐产物的动态运动,阻碍 CPMAS,但可以通过缩短自旋晶格弛豫时间来实现直接 13C 检测。由于固态核磁共振在表征化学吸附反应方面发挥着重要作用,因此这些新见解可以对以前难以捉摸的碳酸氢盐物质(在红外光谱中观察到也具有挑战性)进行常规检测,这代表了一项重要的进步。我们注意到,采用这种简单的 NMR 技术可以揭示碳酸氢盐的存在,而碳酸氢盐通常被忽视,如 APS 所示,人们认为碳酸氢盐仅含有以氨基甲酸盐和氨基甲酸形式吸附的二氧化碳。与其他系统(例如蛋白质)一样,对多种环境进行采样的动态物种往往会随着其运动被冻结而扩大。在这里,我们在冷冻时显示了两种不同的碳酸氢盐物种,并通过初步 13C-1H HETCOR 测量显示了与不同质子的耦合。这项工作表明,碳酸氢盐很可能是在有水的情况下形成的,但由于最常用的实验的性质,核磁共振没有观察到碳酸氢盐,这一观点将改变吸附界看待胺捕获二氧化碳的方式。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号