PLOS Medicine ( IF 10.5 ) Pub Date : 2018-06-26 , DOI: 10.1371/journal.pmed.1002593 Matthew E. Coldiron , Bachir Assao , Anne-Laure Page , Matt D. T. Hitchings , Gabriel Alcoba , Iza Ciglenecki , Céline Langendorf , Christopher Mambula , Eric Adehossi , Fati Sidikou , Elhadji Ibrahim Tassiou , Victoire De Lastours , Rebecca F. Grais
|
Background
Antibiotic prophylaxis for contacts of meningitis cases is not recommended during outbreaks in the African meningitis belt. We assessed the effectiveness of single-dose oral ciprofloxacin administered to household contacts and in village-wide distributions on the overall attack rate (AR) in an outbreak of meningococcal meningitis.
Methods and findings
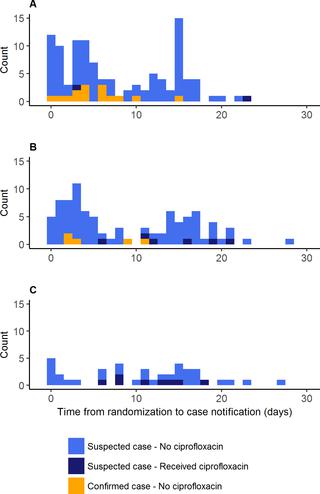
In this 3-arm, open-label, cluster-randomized trial during a meningococcal meningitis outbreak in Madarounfa District, Niger, villages notifying a suspected case were randomly assigned (1:1:1) to standard care (the control arm), single-dose oral ciprofloxacin for household contacts within 24 hours of case notification, or village-wide distribution of ciprofloxacin within 72 hours of first case notification. The primary outcome was the overall AR of suspected meningitis after inclusion. A random sample of 20 participating villages was enrolled to document any changes in fecal carriage prevalence of ciprofloxacin-resistant and extended-spectrum beta-lactamase (ESBL)–producing Enterobacteriaceae before and after the intervention. Between April 22 and May 18, 2017, 49 villages were included: 17 to the control arm, 17 to household prophylaxis, and 15 to village-wide prophylaxis. A total of 248 cases were notified in the study after the index cases. The AR was 451 per 100,000 persons in the control arm, 386 per 100,000 persons in the household prophylaxis arm (t test versus control p = 0.68), and 190 per 100,000 persons in the village-wide prophylaxis arm (t test versus control p = 0.032). The adjusted AR ratio between the household prophylaxis arm and the control arm was 0.94 (95% CI 0.52–1.73, p = 0.85), and the adjusted AR ratio between the village-wide prophylaxis arm and the control arm was 0.40 (95% CI 0.19‒0.87, p = 0.022). No adverse events were notified. Baseline carriage prevalence of ciprofloxacin-resistant Enterobacteriaceae was 95% and of ESBL-producing Enterobacteriaceae was >90%, and did not change post-intervention. One limitation of the study was the small number of cerebrospinal fluid samples sent for confirmatory testing.
Conclusions
Village-wide distribution of single-dose oral ciprofloxacin within 72 hours of case notification reduced overall meningitis AR. Distributions of ciprofloxacin could be an effective tool in future meningitis outbreak responses, but further studies investigating length of protection, effectiveness in urban settings, and potential impact on antimicrobial resistance patterns should be carried out.
Trial registration
ClinicalTrials.gov NCT02724046
中文翻译:

单剂量口服环丙沙星预防作为对非洲脑膜炎带中脑膜炎球菌脑膜炎流行的一种反应:一项三臂,开放标签,整群随机试验
背景
在非洲脑膜炎带爆发期间,不建议对脑膜炎病例进行抗生素预防。我们评估了在一次脑膜炎球菌性脑膜炎暴发中,单剂量口服环丙沙星对家庭接触者和全村分布的总体发作率(AR)的有效性。
方法和发现
在尼日尔Madarounfa区发生脑膜炎球菌性脑膜炎暴发期间的三组,开放标签,整群随机试验中,将通知疑似病例的村庄随机(1:1:1)分配给标准护理(对照组),单组-在病例通报后的24小时内向家庭接触者口服口服环丙沙星,或在首次病例通报后的72小时内在全村分发环丙沙星。主要结局是纳入后疑似脑膜炎的总体AR。随机抽取了20个参与调查的村庄,记录了干预前后大肠环丙沙星耐药和广谱β-内酰胺酶(ESBL)产肠杆菌的粪便携带率的任何变化。在2017年4月22日至5月18日期间,包括49个村庄:17个为对照组,17个为家庭预防,15个村级的预防措施。在索引病例之后,共有248例病例在研究中得到了通知。对照组的AR为每100,000人451,家庭预防组为100,000的386(t检验与对照组的比较p = 0.68),全村预防组每100,000人中有190人(t检验与对照的比较p = 0.032)。家庭预防部门和控制部门之间的调整后AR比率为0.94(95%CI 0.52-1.73,p = 0.85),全村预防部门和控制部门之间的调整后AR比为0.40(95%CI 0.19‒0.87,p = 0.022)。没有不良反应的通知。耐环丙沙星肠杆菌科的基线携带率为95%,产ESBL肠杆菌科的基线携带率为90%以上,干预后未发生变化。该研究的局限性是发送给验证性测试的脑脊液样本数量很少。
结论
病例通报后72小时内,全剂量口服环丙沙星在全村分布减少了整体脑膜炎AR。环丙沙星的分布可能是未来脑膜炎暴发应对的有效工具,但应进行进一步的研究,研究其保护时间,在城市环境中的有效性以及对抗菌素耐药性模式的潜在影响。
试用注册
ClinicalTrials.gov NCT02724046











































 京公网安备 11010802027423号
京公网安备 11010802027423号