Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.3 ) Pub Date : 2018-06-26 , DOI: 10.1016/j.saa.2018.06.084 Brij Mohan , Krunal Modi , Chirag Patel , Pankaj Bhatia , Parveen Kumar , Ashwani Kumar , Harish Kumar Sharma
|
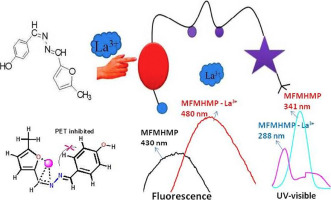
The functionalized molecules with specific molecular sites appear to be a promising approach for detection of cation in UV–visible and fluorescence spectroscopy. The synthesized receptor 4-((5-methylfuran-2-yl)methylene)hydrazono)methyl)phenol MFMHMP was found selective for La3+ among Ag+, K+, Na+, Be2+, Mg2+, Ca2+, Eu3+, Al3+, La3+, Zr4+, Th4+, UO22+, Fe3+, Fe2+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+ and Hg2+ metal ions used as their nitrates by UV–visible spectroscopy and fluorescence spectroscopy. The binding nature of MFMHMP with La3+ ion was analyzed by UV–visible, fluorescence, IR, mass spectroscopy and cyclic voltammetric studies. The stoichiometry was established to be 1:1 by Benesi-Hildebrand, mole-ratio method and method of continuous variation (Job's method) with good association affinity K = 6.245 × 104 M−1. Computational studies and Density functional theory (DFT) calculation gives the proof of electron transfer during excitation and emission. Binding energy of complex through Density Function Theory −62.387 kcal/mol has also indication of strong binding. The electron transfer energy of Higher occupied molecular orbital (HOMO) to Lower unoccupied molecular orbital (LUMO) is about 4.662 eV for MFMHMP+La3+ Complex. Among that all transitions HOMO → LUMO + 8 and HOMO → LUMO + 9 play a key role for the blue shift transition during complexation.
中文翻译:

合成的4-((5-甲基呋喃-2-基)亚甲基)azo基)甲基)酚受体对La 3+离子的选择性及其光谱分析
具有特定分子位点的功能化分子似乎是在紫外可见光谱和荧光光谱中检测阳离子的有前途的方法。发现合成的受体4-(((5-甲基呋喃-2-基)亚甲基)偶氮酰基)甲基)苯酚MFMHMP对La 3+具有选择性,在Ag +,K +,Na +,Be 2 +,Mg 2 +,Ca 2中具有选择性。+,Eu 3+,Al 3+,La 3+,Zr 4+,Th 4+,UO 2 2 +,Fe 3+,Fe 2 +,Co 2 +,Ni 2+紫外可见光谱和荧光光谱法测定了硝酸根中的铜离子,铜离子2+,锌离子2+,镉离子2+和汞离子2+,汞离子2+和汞离子2+的汞离子。MFMHMP与La 3+离子的结合性质通过紫外可见光,荧光,IR,质谱和循环伏安法研究进行了分析。通过Benesi-Hildebrand方法,摩尔比率法和具有良好缔合亲和力的连续变化法(乔布法)将化学计量比确定为1:1,K = 6.245×10 4 M -1。计算研究和密度泛函理论(DFT)计算提供了激发和发射过程中电子转移的证明。通过密度函数理论-62.387 kcal / mol的配合物的结合能也表明了强结合。对于MFMHMP + La 3+配合物,高占据分子轨道(HOMO)到低未占据分子轨道(LUMO)的电子转移能约为4.662 eV 。在所有过渡中,HOMO→LUMO + 8和HOMO→LUMO + 9在复合过程中的蓝移过渡中起关键作用。












































 京公网安备 11010802027423号
京公网安备 11010802027423号