当前位置:
X-MOL 学术
›
Cell. Signal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
RhoA regulates Drp1 mediated mitochondrial fission through ROCK to protect cardiomyocytes.
Cellular Signalling ( IF 4.4 ) Pub Date : 2018-06-25 , DOI: 10.1016/j.cellsig.2018.06.012 Cameron S Brand 1 , Valerie P Tan 1 , Joan Heller Brown 1 , Shigeki Miyamoto 1
Cellular Signalling ( IF 4.4 ) Pub Date : 2018-06-25 , DOI: 10.1016/j.cellsig.2018.06.012 Cameron S Brand 1 , Valerie P Tan 1 , Joan Heller Brown 1 , Shigeki Miyamoto 1
Affiliation
|
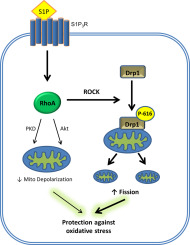
Cardiac ischemia/reperfusion, loss of blood flow and its subsequent restoration, causes damage to the heart. Oxidative stress from ischemia/reperfusion leads to dysfunction and death of cardiomyocytes, increasing the risk of progression to heart failure. Alterations in mitochondrial dynamics, in particular mitochondrial fission, have been suggested to play a role in cardioprotection from oxidative stress. We tested the hypothesis that activation of RhoA regulates mitochondrial fission in cardiomyocytes. Our studies show that expression of constitutively active RhoA in cardiomyocytes increases phosphorylation of Dynamin-related protein 1 (Drp1) at serine-616, and leads to localization of Drp1 at mitochondria. Both responses are blocked by inhibition of Rho-associated Protein Kinase (ROCK). Endogenous RhoA activation by the GPCR agonist sphingosine-1-phosphate (S1P) also increases Drp1 phosphorylation and its mitochondrial translocation in a RhoA and ROCK dependent manner. Consistent with the role of mitochondrial Drp1 in fission, RhoA activation in cardiomyocytes leads to formation of smaller mitochondria and this is attenuated by inhibition of ROCK, by siRNA knockdown of Drp1 or by expression of a phosphorylation-deficient Drp1 S616A mutant. In addition, activation of RhoA prevents cell death in cardiomyocytes challenged by oxidative stress and this protection is blocked by siRNA knockdown of Drp1 or by Drp1 S616A expression. Taken together our findings demonstrate that RhoA activation can regulate Drp1 to induce mitochondrial fission and subsequent cellular protection, implicating regulation of fission as a novel mechanism contributing to RhoA-mediated cardioprotection.
中文翻译:

RhoA通过ROCK调节Drp1介导的线粒体裂变,以保护心肌细胞。
心脏缺血/再灌注,血流损失及其随后的恢复会损害心脏。缺血/再灌注引起的氧化应激导致心肌细胞功能障碍和死亡,从而增加了发展为心力衰竭的风险。线粒体动力学的变化,特别是线粒体裂变,已被证明在心脏保护免受氧化应激中起着作用。我们测试了RhoA激活调节心肌细胞线粒体分裂的假说。我们的研究表明,心肌细胞中组成型活性RhoA的表达增加了丝氨酸616上的Dynamin相关蛋白1(Drp1)的磷酸化,并导致Drp1定位于线粒体。两种反应都被Rho相关蛋白激酶(ROCK)的抑制所阻断。由GPCR激动剂-1-磷酸鞘氨醇(S1P)激活的内源性RhoA也以RhoA和ROCK依赖性方式增加Drp1磷酸化及其线粒体易位。与线粒体Drp1在裂变中的作用一致,心肌细胞中的RhoA活化导致形成较小的线粒体,并通过抑制ROCK,通过siRNA敲低Drp1或通过表达磷酸化缺陷的Drp1 S616A突变体而减弱。此外,RhoA的激活可防止心肌细胞受到氧化应激攻击而死亡,并且这种保护作用可通过敲除Drp1的siRNA或Drp1 S616A的表达来阻断。综上所述,我们的发现表明RhoA激活可以调节Drp1诱导线粒体裂变和随后的细胞保护,
更新日期:2018-06-25
中文翻译:

RhoA通过ROCK调节Drp1介导的线粒体裂变,以保护心肌细胞。
心脏缺血/再灌注,血流损失及其随后的恢复会损害心脏。缺血/再灌注引起的氧化应激导致心肌细胞功能障碍和死亡,从而增加了发展为心力衰竭的风险。线粒体动力学的变化,特别是线粒体裂变,已被证明在心脏保护免受氧化应激中起着作用。我们测试了RhoA激活调节心肌细胞线粒体分裂的假说。我们的研究表明,心肌细胞中组成型活性RhoA的表达增加了丝氨酸616上的Dynamin相关蛋白1(Drp1)的磷酸化,并导致Drp1定位于线粒体。两种反应都被Rho相关蛋白激酶(ROCK)的抑制所阻断。由GPCR激动剂-1-磷酸鞘氨醇(S1P)激活的内源性RhoA也以RhoA和ROCK依赖性方式增加Drp1磷酸化及其线粒体易位。与线粒体Drp1在裂变中的作用一致,心肌细胞中的RhoA活化导致形成较小的线粒体,并通过抑制ROCK,通过siRNA敲低Drp1或通过表达磷酸化缺陷的Drp1 S616A突变体而减弱。此外,RhoA的激活可防止心肌细胞受到氧化应激攻击而死亡,并且这种保护作用可通过敲除Drp1的siRNA或Drp1 S616A的表达来阻断。综上所述,我们的发现表明RhoA激活可以调节Drp1诱导线粒体裂变和随后的细胞保护,

































 京公网安备 11010802027423号
京公网安备 11010802027423号