当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Human HSP70 Promoter‐Based Prussian Blue Nanotheranostics for Thermo‐Controlled Gene Therapy and Synergistic Photothermal Ablation
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2018-06-21 , DOI: 10.1002/adfm.201802026
Yajuan Liu 1 , Guiming Shu 2 , Xue Li 3 , Hongbin Chen 1 , Bo Zhang 1 , Huizhuo Pan 1 , Tao Li 2 , Xiaoqun Gong 1 , Hanjie Wang 1 , Xiaoli Wu 1 , Yan Dou 4 , Jin Chang 1
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2018-06-21 , DOI: 10.1002/adfm.201802026
Yajuan Liu 1 , Guiming Shu 2 , Xue Li 3 , Hongbin Chen 1 , Bo Zhang 1 , Huizhuo Pan 1 , Tao Li 2 , Xiaoqun Gong 1 , Hanjie Wang 1 , Xiaoli Wu 1 , Yan Dou 4 , Jin Chang 1
Affiliation
|
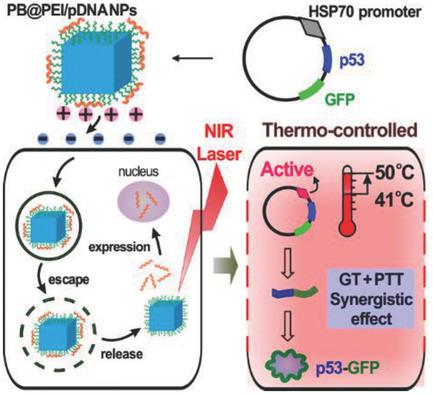
Realizing precise control of the therapeutic process is crucial for maximizing efficacy and minimizing side effects, especially for strategies involving gene therapy (GT). Herein, a multifunctional Prussian blue (PB) nanotheranostic platform is first designed and then loaded with therapeutic plasmid DNA (HSP70‐p53‐GFP) for near‐infrared (NIR) light‐triggered thermo‐controlled synergistic GT/photothermal therapy (PTT). Due to the unique structure of the PB nanocubes, the resulting PB@PEI/HSP70‐p53‐GFP nanoparticles (NPs) exhibit excellent photothermal properties and pronounced tumor‐contrast performance in T1/T2‐weighted magnetic resonance imaging. Both in vitro and in vivo studies demonstrate that mild NIR‐laser irradiation (≈41 °C) activates the HSP70 promoter for tumor suppressor p53‐dependent apoptosis, while strong NIR‐laser irradiation (≈50 °C) induces photothermal ablation for cellular dysregulation and necrosis. Significant synergistic efficacy can be achieved by adjusting the NIR‐laser irradiation (from ≈41 to ≈50 °C), compared to using GT or PTT alone. In addition, in vitro and in vivo toxicity studies demonstrate that PB@PEI/HSP70‐p53‐GFP NPs have good biocompatibility. Therefore, this work provides a promising theranostic approach for controlling combined GT and PTT via the heat‐shock response.
中文翻译:

基于HSP70启动子的普鲁士蓝Nanotheranostics用于热控制基因治疗和协同光热消融。
实现治疗过程的精确控制对于最大化功效和最小化副作用至关重要,特别是对于涉及基因治疗(GT)的策略。本文首先设计了多功能的普鲁士蓝(PB)纳米热平台,然后装载了治疗性质粒DNA(HSP70-p53-GFP),用于近红外(NIR)光触发的热控协同GT /光热疗法(PTT)。由于PB纳米立方体的独特结构,所得的PB @ PEI / HSP70-p53-GFP纳米颗粒(NP)在T 1 / T 2中表现出出色的光热性能和明显的肿瘤对比性能加权磁共振成像。体内外研究均表明,轻度NIR激光照射(≈41°C)激活HSP70启动子以抑制p53依赖的肿瘤细胞凋亡,而强NIR激光照射(≈50°C)诱导光热消融,引起细胞失调。和坏死。与单独使用GT或PTT相比,通过调节NIR激光辐射(从≈41到≈50°C)可以实现显着的协同功效。此外,体外和体内毒性研究表明PB @ PEI / HSP70-p53-GFP NP具有良好的生物相容性。因此,这项工作为通过热冲击响应控制GT和PTT结合提供了一种有前景的治疗方法。
更新日期:2018-06-21
中文翻译:

基于HSP70启动子的普鲁士蓝Nanotheranostics用于热控制基因治疗和协同光热消融。
实现治疗过程的精确控制对于最大化功效和最小化副作用至关重要,特别是对于涉及基因治疗(GT)的策略。本文首先设计了多功能的普鲁士蓝(PB)纳米热平台,然后装载了治疗性质粒DNA(HSP70-p53-GFP),用于近红外(NIR)光触发的热控协同GT /光热疗法(PTT)。由于PB纳米立方体的独特结构,所得的PB @ PEI / HSP70-p53-GFP纳米颗粒(NP)在T 1 / T 2中表现出出色的光热性能和明显的肿瘤对比性能加权磁共振成像。体内外研究均表明,轻度NIR激光照射(≈41°C)激活HSP70启动子以抑制p53依赖的肿瘤细胞凋亡,而强NIR激光照射(≈50°C)诱导光热消融,引起细胞失调。和坏死。与单独使用GT或PTT相比,通过调节NIR激光辐射(从≈41到≈50°C)可以实现显着的协同功效。此外,体外和体内毒性研究表明PB @ PEI / HSP70-p53-GFP NP具有良好的生物相容性。因此,这项工作为通过热冲击响应控制GT和PTT结合提供了一种有前景的治疗方法。































 京公网安备 11010802027423号
京公网安备 11010802027423号