当前位置:
X-MOL 学术
›
Arch. Pharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Antileishmanial activity, structure-activity relationship of series of 2-(trifluoromethyl)benzo[b ][1,8]naphthyridin-4(1H )-ones
Archiv der Pharmazie ( IF 4.3 ) Pub Date : 2018-06-21 , DOI: 10.1002/ardp.201800094 Angel H Romero 1 , Simon E López 2 , Noris Rodríguez 1 , Henry Oviedo 1
Archiv der Pharmazie ( IF 4.3 ) Pub Date : 2018-06-21 , DOI: 10.1002/ardp.201800094 Angel H Romero 1 , Simon E López 2 , Noris Rodríguez 1 , Henry Oviedo 1
Affiliation
|
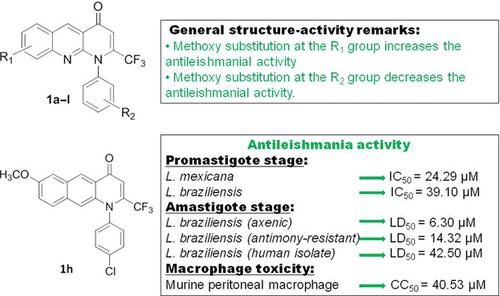
Trifluoromethyl‐substituted quinolones and their analogues have emerged as an interesting platform in the last 6 years to design antiparasite agents. Many of their derivatives have been demonstrated to display excellent efficacy against flagellate parasites such as Plasmodium spp. In order to identify new analogues of trifluoromethyl‐substituted quinolones to treat the American cutaneous leishmaniasis, we evaluated the antiproliferative activity of a series of 2‐(trifluoromethyl)benzo[b]‐[1,8]naphthyridin‐4(1H)‐ones on the Leishmania braziliensis and Leishmania mexicana parasites. The mentioned derivatives have never been evaluated against any parasite strain. In general, an in vitro evaluation on L.(L)mexicana and L.(V)braziliensis showed that L.(L)mexicana was more sensitive to the action of the compounds than L.(V)braziliensis, either in the promastigote or in the amastigote form. Five compounds exhibited moderate efficacy against L.(L)mexicana promastigotes, with IC50 values ranging from 9.65 to 14.76 µM. From the mentioned molecules, three compounds, 1e, 1f, and 1h, showed a discrete response against axenic and intracellular amastigotes, with LD50 values between 19 and 24 µM. Moreover, an in vitro evaluation was performed on an antimony‐resistant amastigote strain and a human isolate amastigote strain. These three compounds showed discrete toxicity on peritoneal macrophages; however, their relatively good antiamastigote response compared to the drug glucantime promoted our trifluoromethyl‐substituted benzo[b][1,8]naphthyridin‐4(1H)‐ones as a potential platform to design potent antileishmanial agents.
中文翻译:

2-(三氟甲基)苯并[b][1,8]萘啶-4(1H)-酮系列的抗利什曼原虫活性、构效关系
在过去的六年里,三氟甲基取代的喹诺酮类药物及其类似物已成为设计抗寄生虫药物的一个有趣的平台。它们的许多衍生物已被证明对鞭毛虫寄生虫(如疟原虫属)表现出优异的功效。为了确定三氟甲基取代喹诺酮类药物的新类似物来治疗美国皮肤利什曼病,我们评估了一系列 2-(三氟甲基)苯并[b]-[1,8]萘啶-4(1H)-酮的抗增殖活性巴西利什曼原虫和墨西哥利什曼原虫寄生虫。上述衍生物从未针对任何寄生虫菌株进行过评估。一般而言,对 L.(L)mexicana 和 L.(V)braziliensis 的体外评估表明,无论是在前鞭毛体中,L.(L)mexicana 比 L.(V)braziliensis 对化合物的作用更敏感或以无鞭毛体形式。五种化合物对 L.(L)mexicana 前鞭毛体表现出中等功效,IC50 值范围为 9.65 至 14.76 µM。从上述分子中,三种化合物 1e、1f 和 1h 对无菌和细胞内无鞭毛体表现出离散反应,LD50 值在 19 至 24 µM 之间。此外,对抗锑无鞭毛体菌株和人分离无鞭毛体菌株进行了体外评估。这三种化合物对腹膜巨噬细胞表现出不同的毒性;然而,与药物葡聚糖相比,它们相对较好的抗鞭毛体反应促进了我们的三氟甲基取代的苯并[b][1,8]萘啶-4(1H)-酮作为设计有效的抗利什曼病药物的潜在平台。
更新日期:2018-06-21
中文翻译:

2-(三氟甲基)苯并[b][1,8]萘啶-4(1H)-酮系列的抗利什曼原虫活性、构效关系
在过去的六年里,三氟甲基取代的喹诺酮类药物及其类似物已成为设计抗寄生虫药物的一个有趣的平台。它们的许多衍生物已被证明对鞭毛虫寄生虫(如疟原虫属)表现出优异的功效。为了确定三氟甲基取代喹诺酮类药物的新类似物来治疗美国皮肤利什曼病,我们评估了一系列 2-(三氟甲基)苯并[b]-[1,8]萘啶-4(1H)-酮的抗增殖活性巴西利什曼原虫和墨西哥利什曼原虫寄生虫。上述衍生物从未针对任何寄生虫菌株进行过评估。一般而言,对 L.(L)mexicana 和 L.(V)braziliensis 的体外评估表明,无论是在前鞭毛体中,L.(L)mexicana 比 L.(V)braziliensis 对化合物的作用更敏感或以无鞭毛体形式。五种化合物对 L.(L)mexicana 前鞭毛体表现出中等功效,IC50 值范围为 9.65 至 14.76 µM。从上述分子中,三种化合物 1e、1f 和 1h 对无菌和细胞内无鞭毛体表现出离散反应,LD50 值在 19 至 24 µM 之间。此外,对抗锑无鞭毛体菌株和人分离无鞭毛体菌株进行了体外评估。这三种化合物对腹膜巨噬细胞表现出不同的毒性;然而,与药物葡聚糖相比,它们相对较好的抗鞭毛体反应促进了我们的三氟甲基取代的苯并[b][1,8]萘啶-4(1H)-酮作为设计有效的抗利什曼病药物的潜在平台。

































 京公网安备 11010802027423号
京公网安备 11010802027423号