当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Synthetic Light-Driven Substrate Channeling System for Precise Regulation of Enzyme Cascade Activity Based on DNA Origami
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-06-21 , DOI: 10.1021/jacs.8b05429 Yahong Chen 1 , Guoliang Ke 2 , Yanli Ma 1 , Zhi Zhu 1 , Minghui Liu 3 , Yan Liu 3 , Hao Yan 3 , Chaoyong James Yang 1, 4
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-06-21 , DOI: 10.1021/jacs.8b05429 Yahong Chen 1 , Guoliang Ke 2 , Yanli Ma 1 , Zhi Zhu 1 , Minghui Liu 3 , Yan Liu 3 , Hao Yan 3 , Chaoyong James Yang 1, 4
Affiliation
|
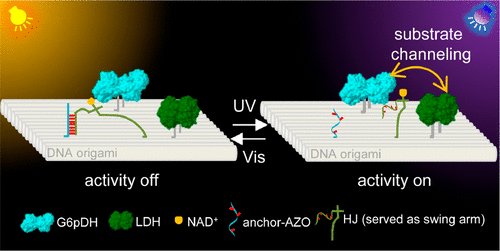
Substrate channeling, in which a metabolic intermediate is directly passed from one enzyme to the next enzyme in an enzyme cascade, accelerates the processing of metabolites and improves substrate selectivity. Synthetic design and precise control of channeling outside the cellular environment are of significance in areas such as synthetic biology, synthetic chemistry, and biomedicine. In particular, the precise control of synthetic substrate channeling in response to light is highly important, but remains a major challenge. Herein, we develop a photoresponsive molecule-based synthetic substrate channeling system on DNA origami to regulate enzyme cascade activity. The photoresponsive azobenzene molecules introduced into DNA strands enable reversible switching of the position of substrate channeling to selectively activate or inhibit the enzyme cascade activity. Moreover, DNA origami allows precise control of interenzyme distance and swinging range of the swing arm to optimize the regulation efficiency. By combining the accurate and addressable assembly ability of DNA origami and the clean, rapid, and reversible regulation of photoresponsive molecules, this light-driven substrate channeling system is expected to find important applications in synthetic biology and biomedicine.
中文翻译:

基于 DNA Origami 的用于精确调控酶级联活性的合成光驱动底物通道系统
底物通道,其中代谢中间体在酶级联中直接从一种酶传递到下一种酶,加速代谢物的加工并提高底物选择性。细胞环境外通道的合成设计和精确控制在合成生物学、合成化学和生物医学等领域具有重要意义。特别是,响应光的合成底物通道的精确控制非常重要,但仍然是一个重大挑战。在此,我们在 DNA 折纸上开发了一种基于光响应分子的合成底物通道系统,以调节酶级联活性。引入 DNA 链的光响应偶氮苯分子能够可逆地转换底物通道的位置,从而选择性地激活或抑制酶级联活性。此外,DNA折纸可以精确控制酶间距离和摆臂的摆动范围,以优化调节效率。通过将 DNA 折纸的准确和可寻址组装能力与光响应分子的清洁、快速和可逆调节相结合,这种光驱动的底物通道系统有望在合成生物学和生物医学中找到重要应用。
更新日期:2018-06-21
中文翻译:

基于 DNA Origami 的用于精确调控酶级联活性的合成光驱动底物通道系统
底物通道,其中代谢中间体在酶级联中直接从一种酶传递到下一种酶,加速代谢物的加工并提高底物选择性。细胞环境外通道的合成设计和精确控制在合成生物学、合成化学和生物医学等领域具有重要意义。特别是,响应光的合成底物通道的精确控制非常重要,但仍然是一个重大挑战。在此,我们在 DNA 折纸上开发了一种基于光响应分子的合成底物通道系统,以调节酶级联活性。引入 DNA 链的光响应偶氮苯分子能够可逆地转换底物通道的位置,从而选择性地激活或抑制酶级联活性。此外,DNA折纸可以精确控制酶间距离和摆臂的摆动范围,以优化调节效率。通过将 DNA 折纸的准确和可寻址组装能力与光响应分子的清洁、快速和可逆调节相结合,这种光驱动的底物通道系统有望在合成生物学和生物医学中找到重要应用。






























 京公网安备 11010802027423号
京公网安备 11010802027423号