当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Generation of a double transgenic humanized neonatal Fc receptor (FcRn)/albumin mouse to study the pharmacokinetics of albumin-linked drugs
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2015-12-25 05:11:29
Dorthe Viuff, Filipa Antunes, Leslie Evans, Jason Cameron, Hans Dyrnesli, Birgitte Thue Ravn, Magnus Stougaard, Kader Thiam, Birgitte Andersen, Søren Kjærulff, Kenneth A. Howard
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2015-12-25 05:11:29
Dorthe Viuff, Filipa Antunes, Leslie Evans, Jason Cameron, Hans Dyrnesli, Birgitte Thue Ravn, Magnus Stougaard, Kader Thiam, Birgitte Andersen, Søren Kjærulff, Kenneth A. Howard
|
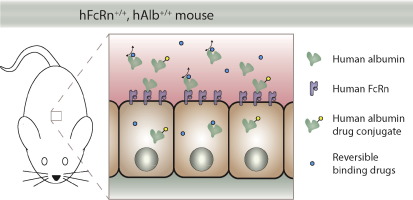
Human serum albumin (HSA) is a natural carrier protein possessing multiple ligand binding sites with a plasma half-life ~19days, facilitated by interaction with the human neonatal Fc receptor (FcRn), that promotes it as a highly attractive drug delivery technology. A lack of adequate rodent models, however, is a major challenge in the preclinical development of albumin-linked therapeutics. This work describes the first double transgenic mouse model bearing both human FcRn and HSA genes (hFcRn+/+, hAlb+/+) under the control of an endogenous promoter. Human FcRn was shown by immunohistochemical and qPCR analysis to be ubiquitously expressed in the major organs. Physiological levels of HSA were detected in the blood that exhibited similar FcRn binding kinetics to recombinant or human serum-derived HSA. The circulatory half-life (t1/2) was shown to be dependent on FcRn binding affinity that increased from low affinity (t1/2 29h), to wild type (t1/2 50h), to high affinity (t1/2 80h) variants, that validates the application of the model for optimizing the pharmacokinetics of drug carriers who's circulatory half-life is dependent in some manner upon interaction with endogenous FcRn. This study presents a novel mouse model that better mimics the human physiological conditions and, thus, has potential wide applications in the development of albumin-linked drugs or conventional drugs whose action is influenced by reversible binding to endogenous HSA.
中文翻译:

产生双转基因人源化新生儿Fc受体(FcRn)/白蛋白小鼠以研究与白蛋白相关的药物的药代动力学
人血清白蛋白(HSA)是一种天然载体蛋白,具有多个配体结合位点,血浆半衰期约为19天,与人新生儿Fc受体(FcRn)相互作用促进了这种活性,将其促进为高度有吸引力的药物递送技术。然而,缺乏合适的啮齿动物模型是与白蛋白相关的治疗剂的临床前开发中的主要挑战。这项工作描述了第一个同时携带人FcRn和HSA基因(hFcRn + / +,hAlb + / +)在内源性启动子的控制下。免疫组化和qPCR分析显示人FcRn在主要器官中普遍表达。在血液中检测到HSA的生理水平,这些血液显示出与重组或人血清来源的HSA相似的FcRn结合动力学。循环半衰期(t 1/2)依赖于FcRn结合亲和力,从低亲和力(t 1/2 29h)到野生型(t 1/2 50h)到高亲和力(t 1 / 280h)变体,验证了该模型在优化循环半衰期药物载体的药代动力学中的应用,在某种程度上取决于与内源性FcRn的相互作用。这项研究提出了一种新型的小鼠模型,可以更好地模拟人类的生理状况,因此,在白蛋白链接药物或常规药物的开发中具有潜在的广泛应用,这些药物的作用受与内源性HSA的可逆结合影响。
更新日期:2015-12-26
中文翻译:

产生双转基因人源化新生儿Fc受体(FcRn)/白蛋白小鼠以研究与白蛋白相关的药物的药代动力学
人血清白蛋白(HSA)是一种天然载体蛋白,具有多个配体结合位点,血浆半衰期约为19天,与人新生儿Fc受体(FcRn)相互作用促进了这种活性,将其促进为高度有吸引力的药物递送技术。然而,缺乏合适的啮齿动物模型是与白蛋白相关的治疗剂的临床前开发中的主要挑战。这项工作描述了第一个同时携带人FcRn和HSA基因(hFcRn + / +,hAlb + / +)在内源性启动子的控制下。免疫组化和qPCR分析显示人FcRn在主要器官中普遍表达。在血液中检测到HSA的生理水平,这些血液显示出与重组或人血清来源的HSA相似的FcRn结合动力学。循环半衰期(t 1/2)依赖于FcRn结合亲和力,从低亲和力(t 1/2 29h)到野生型(t 1/2 50h)到高亲和力(t 1 / 280h)变体,验证了该模型在优化循环半衰期药物载体的药代动力学中的应用,在某种程度上取决于与内源性FcRn的相互作用。这项研究提出了一种新型的小鼠模型,可以更好地模拟人类的生理状况,因此,在白蛋白链接药物或常规药物的开发中具有潜在的广泛应用,这些药物的作用受与内源性HSA的可逆结合影响。

































 京公网安备 11010802027423号
京公网安备 11010802027423号