当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A comparative study on the oxidation of two-dimensional Ti3C2 MXene structures in different environments
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2018-06-20 00:00:00 , DOI: 10.1039/c8ta01468j
Roghayyeh Lotfi 1, 2, 3, 4 , Michael Naguib 4, 5, 6, 7 , Dundar E. Yilmaz 1, 2, 3, 4 , Jagjit Nanda 4, 5, 6, 7 , Adri C. T. van Duin 1, 2, 3, 4
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2018-06-20 00:00:00 , DOI: 10.1039/c8ta01468j
Roghayyeh Lotfi 1, 2, 3, 4 , Michael Naguib 4, 5, 6, 7 , Dundar E. Yilmaz 1, 2, 3, 4 , Jagjit Nanda 4, 5, 6, 7 , Adri C. T. van Duin 1, 2, 3, 4
Affiliation
|
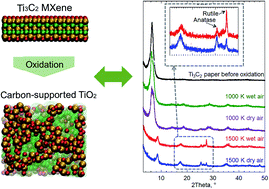
The oxidation of two-dimensional Ti3C2 MXene structures has been recognized as a promising method for the formation of hybrid structures of carbon supported nanotitania. Here, we studied the oxidation of Ti3C2 MXene structures using reactive force field (ReaxFF) based molecular dynamics simulations. To investigate the effect of oxidizing agent, we used three different environments including dry air (oxygen molecules), wet air (oxygen and water molecules) and hydrogen peroxide (H2O2 molecules). Oxidation simulations were performed at different temperatures of 1000, 1500, 2000, 2500 and 3000 K. We found that by controlling the temperature, carbon supported titania can be formed by diffusion of Ti atoms to the surface of MXene structures during the oxidation. These results were confirmed by the increase in the average bond orders of C–C and Ti–O and decrease in the average bond orders of Ti–C. Moreover, it was revealed that by increasing the temperature, the oxidation rate increases, and the rate depends on the oxidant according to the following order: H2O2 > wet air > dry air. This was validated by heating the MXene in wet air and dry air followed by examining the products using X-ray diffraction and Raman spectroscopy. To investigate the effect of the presence of the oxidant, the MXene structure was also heated under vacuum, and it was found that in this case the MXene structure was converted to cubic TiC. Furthermore, it was indicated that during the heating process of the MXene structure in a vacuum, the average bond orders for C–C, Ti–C and Ti–O do not change significantly due to the lack of oxidants in the environment, leaving room for only topotactic transformation of atoms during the heating. The formation of the TiC structure by heating the MXene in an inert environment was confirmed experimentally as well.
中文翻译:

二维Ti 3 C 2 MXene结构在不同环境下 氧化的比较研究。
二维Ti 3 C 2 MXene结构的氧化被认为是形成碳负载纳米二氧化钛杂化结构的有前途的方法。在这里,我们使用基于反作用力场(ReaxFF)的分子动力学模拟研究了Ti 3 C 2 MXene结构的氧化。为了研究氧化剂的作用,我们使用了三种不同的环境,包括干燥空气(氧气分子),潮湿空气(氧气和水分子)和过氧化氢(H 2 O 2)分子)。在1000、1500、2000、2500和3000 K的不同温度下进行了氧化模拟。我们发现,通过控制温度,可以在氧化过程中通过将Ti原子扩散到MXene结构的表面来形成碳负载的二氧化钛。这些结果被C–C和Ti–O的平均债券订单数量的增加和Ti–C的平均债券订单数量的减少所证实。此外,揭示了通过升高温度,氧化速率增加,并且该速率取决于氧化剂,其顺序如下:H 2 O 2。>湿空气>干空气。通过在湿空气和干空气中加热MXene,然后使用X射线衍射和拉曼光谱检查产品来验证这一点。为了研究氧化剂的存在的影响,还在真空下加热了MXene结构,并且发现在这种情况下,MXene结构被转化为立方TiC。此外,还表明,在真空条件下加热MXene结构的过程中,由于环境中缺乏氧化剂,CC,Ti-C和Ti-O的平均键序不会显着变化。仅用于加热过程中原子的定势转变。还通过实验证实了通过在惰性环境中加热MXene而形成的TiC结构。
更新日期:2018-06-20
中文翻译:

二维Ti 3 C 2 MXene结构在不同环境下 氧化的比较研究。
二维Ti 3 C 2 MXene结构的氧化被认为是形成碳负载纳米二氧化钛杂化结构的有前途的方法。在这里,我们使用基于反作用力场(ReaxFF)的分子动力学模拟研究了Ti 3 C 2 MXene结构的氧化。为了研究氧化剂的作用,我们使用了三种不同的环境,包括干燥空气(氧气分子),潮湿空气(氧气和水分子)和过氧化氢(H 2 O 2)分子)。在1000、1500、2000、2500和3000 K的不同温度下进行了氧化模拟。我们发现,通过控制温度,可以在氧化过程中通过将Ti原子扩散到MXene结构的表面来形成碳负载的二氧化钛。这些结果被C–C和Ti–O的平均债券订单数量的增加和Ti–C的平均债券订单数量的减少所证实。此外,揭示了通过升高温度,氧化速率增加,并且该速率取决于氧化剂,其顺序如下:H 2 O 2。>湿空气>干空气。通过在湿空气和干空气中加热MXene,然后使用X射线衍射和拉曼光谱检查产品来验证这一点。为了研究氧化剂的存在的影响,还在真空下加热了MXene结构,并且发现在这种情况下,MXene结构被转化为立方TiC。此外,还表明,在真空条件下加热MXene结构的过程中,由于环境中缺乏氧化剂,CC,Ti-C和Ti-O的平均键序不会显着变化。仅用于加热过程中原子的定势转变。还通过实验证实了通过在惰性环境中加热MXene而形成的TiC结构。

































 京公网安备 11010802027423号
京公网安备 11010802027423号