当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Novel 3,4-Chloroisothiazole-Based Imidazoles as Fungicides and Evaluation of Their Mode of Action
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2018-06-18 00:00:00 , DOI: 10.1021/acs.jafc.8b02332 Lai Chen 1 , Bin Zhao 1 , Zhijin Fan 1, 2 , Xiumei Liu 1 , Qifan Wu 1 , Hongpeng Li 1 , Haixia Wang 1
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2018-06-18 00:00:00 , DOI: 10.1021/acs.jafc.8b02332 Lai Chen 1 , Bin Zhao 1 , Zhijin Fan 1, 2 , Xiumei Liu 1 , Qifan Wu 1 , Hongpeng Li 1 , Haixia Wang 1
Affiliation
|
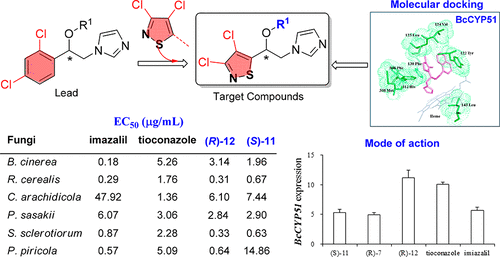
A molecular design approach was used in our laboratory to guide the development of imidazole-based fungicides. Based on homology modeling and molecular docking studies targeting the cytochrome P450-dependent sterol 14α-demethylase, 3,4-dichloroisothiazole-based imidazoles showed great potential. Several such compounds were then rationally designed, synthesized, characterized, and their antifungal activities were evaluated. Bioassay results showed that compounds such as (R)-11, (R)-12, and (S)-11 have commendable, broad-spectrum antifungal activities that are comparable to those of commercial products. Based on Q-PCR testing and microscopy observations, the imidazole derivatives affect fungal cell wall formation through the inhibition of the BcCYP51 expression system. These findings strongly suggest that the mode of action of these imidazole compounds is similar to that of tioconazole and imazalil. This report indicates that this molecular design strategy is not only practical but productive.
中文翻译:

新型3,4-氯异噻唑基咪唑类化合物的合成及其作用方式的评价
在我们的实验室中使用了一种分子设计方法来指导咪唑类杀菌剂的开发。基于针对细胞色素P450依赖的固醇14α-脱甲基酶的同源性建模和分子对接研究,基于3,4-二氯异噻唑的咪唑显示出巨大的潜力。然后合理地设计,合成,表征了几种此类化合物,并评估了它们的抗真菌活性。生物测定结果表明,化合物(R)-11,(R)-12和(S)-11具有与商业产品相当的值得赞扬的广谱抗真菌活性。基于Q-PCR测试和显微镜观察,咪唑衍生物通过抑制BcCYP51表达系统影响真菌细胞壁的形成。这些发现强烈表明这些咪唑化合物的作用方式类似于噻康唑和咪唑的作用方式。该报告表明,这种分子设计策略不仅实用,而且富有成效。
更新日期:2018-06-18
中文翻译:

新型3,4-氯异噻唑基咪唑类化合物的合成及其作用方式的评价
在我们的实验室中使用了一种分子设计方法来指导咪唑类杀菌剂的开发。基于针对细胞色素P450依赖的固醇14α-脱甲基酶的同源性建模和分子对接研究,基于3,4-二氯异噻唑的咪唑显示出巨大的潜力。然后合理地设计,合成,表征了几种此类化合物,并评估了它们的抗真菌活性。生物测定结果表明,化合物(R)-11,(R)-12和(S)-11具有与商业产品相当的值得赞扬的广谱抗真菌活性。基于Q-PCR测试和显微镜观察,咪唑衍生物通过抑制BcCYP51表达系统影响真菌细胞壁的形成。这些发现强烈表明这些咪唑化合物的作用方式类似于噻康唑和咪唑的作用方式。该报告表明,这种分子设计策略不仅实用,而且富有成效。






























 京公网安备 11010802027423号
京公网安备 11010802027423号