当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of 8-substituted-6-phenyl-6,7,8,9-tetrahydro-3H-pyrazolo[4,3-f]isoquinolines using Pictet-Spengler and Bischler-Napieralski cyclisation methods
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2018-06-18 , DOI: 10.1016/j.tetlet.2018.06.041 Robert D.M. Davies , Jennifer H. Pink , James S. Scott , Andrew Bailey
中文翻译:

利用Pictet-Spengler和Bischler-Napieralski环化方法合成8-取代的6-苯基-6,7,8,9-四氢-3 H-吡唑并[4,3- f ]异喹啉
更新日期:2018-06-18
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2018-06-18 , DOI: 10.1016/j.tetlet.2018.06.041 Robert D.M. Davies , Jennifer H. Pink , James S. Scott , Andrew Bailey
|
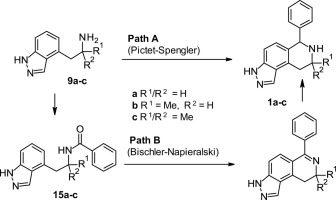
In the following communication we report routes for the synthesis of a set of 8-substituted-6-phenyl-6,7,8,9-tetrahydro-3H-pyrazolo[4,3-f]isoquinolines. Pictet-Spengler and Bischler-Napieralski methodologies were employed on the relevant indazole precursors and the merits of the two cyclisation reactions for preparing these structures were assessed.
中文翻译:

利用Pictet-Spengler和Bischler-Napieralski环化方法合成8-取代的6-苯基-6,7,8,9-四氢-3 H-吡唑并[4,3- f ]异喹啉
在下面的通讯中,我们报告了一组8-取代的6-苯基-6,7,8,9-四氢-3 H-吡唑并[4,3- f ]异喹啉的合成路线。在相关的吲唑前体上使用了Pictet-Spengler和Bischler-Napieralski方法,并评估了制备这些结构的两个环化反应的优缺点。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号