Chemosphere ( IF 8.1 ) Pub Date : 2018-06-15 , DOI: 10.1016/j.chemosphere.2018.06.104 Zhiwei Zhao , Cong Geng , Chun Yang , Fuyi Cui , Zhijie Liang
|
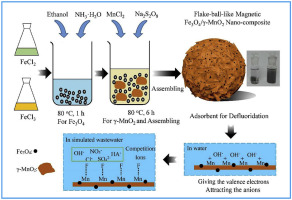
A novel flake-ball-like magnetic Fe3O4/γ-MnO2 meso-porous nano-composite was synthesized and characterized for defluoridation. Adsorption process, characters, and effects of solution chemistry on the adsorption of flourinion in Fe3O4/γ-MnO2 were evaluated. The results show that the adsorption of fluorinion in the Fe3O4/γ-MnO2 nano-composite is fitted with the Pseudo-first model and the Langmuir model, indicating that the adsorption process of fluorinion in the Fe3O4/γ-MnO2 nano-composite was a physical process and not only controlled by the film diffusion but also controlled by the intra-particle diffusion and surface adsorption. It shows that the adsorption of fluorinion sharply decrease with the increase of pH due to the negative changed surface of Fe3O4/γ-MnO2 in water and the competition of OH− for the active points. The competition from decreases the adsorption of fluoride in the order of Cl− < NO3− < SO42−, which relied on the ratio of charge towards radius (z/r) of the anions, and the negatively charged humic acid competed with fluorinion for the adsorption sites. Based on the adsorption results and the XPS analysis, the O
Mn bond in the raw adsorbent supported the active site (O Mn
Mn OH) for fluoride adsorption by forming an O
OH) for fluoride adsorption by forming an O Mn
Mn F bond on the surface of Fe3O4/γ-MnO2.
F bond on the surface of Fe3O4/γ-MnO2.
中文翻译:

一种新的薄片状球状的磁性的Fe 3 ö 4 /γ-MnO的2介孔纳米复合材料:氟离子的吸附和水化学的效果
一种新的薄片状球状的磁性的Fe 3 ö 4 /γ-MnO的2介孔纳米复合材料的合成和表征为除氟。吸附过程,字符和上flourinion的以Fe吸附溶液化学的影响3 ö 4 /γ-MnO的2进行了评价。结果表明,氟离子的在Fe的吸附3 ö 4 /γ-MnO的2纳米复合材料装配有所述伪第一模型和Langmuir模型,表明氟离子在吸附过程中的Fe 3 ö 4 /γ -MnO 2纳米复合材料是一个物理过程,不仅受薄膜扩散的控制,而且还受粒子内扩散和表面吸附的控制。它表明,氟离子的吸附急剧随着pH的增加而降低,由于Fe的负变表面3 ö 4 /γ-MnO的2在水和OH的竞争-为活性点。从竞争减小的氟化物中的Cl的顺序吸附- <NO 3 - <SO 4 2-,这取决于阴离子的电荷与半径之比(z / r),带负电荷的腐殖酸与氟竞争吸附位点。根据吸附结果和XPS分析,O
原始吸附剂中的Mn键支撑了活性位点(O 锰
锰 OH)通过形成O来吸附氟化物
OH)通过形成O来吸附氟化物 锰
锰 Fe3O4 /γ-MnO2表面的F键。
Fe3O4 /γ-MnO2表面的F键。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号