当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Visible Photocatalytic Water Splitting and Photocatalytic Two-Electron Oxygen Formation over Cu- and Fe-Doped g-C3N4
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2015-12-22 00:00:00 , DOI: 10.1021/acs.jpcc.5b09469 Zhen Li 1, 2 , Chao Kong 1, 2 , Gongxuan Lu 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2015-12-22 00:00:00 , DOI: 10.1021/acs.jpcc.5b09469 Zhen Li 1, 2 , Chao Kong 1, 2 , Gongxuan Lu 1
Affiliation
|
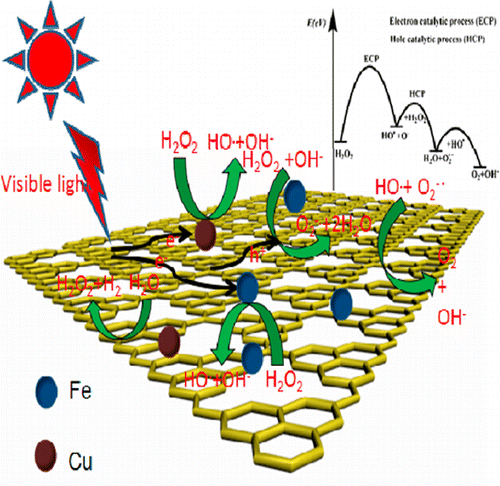
Water splitting via two two-electron processes (the H2O first photocatalytically converted to H2 and H2O2 under visible light irradiation and then the H2O2 disproportionation to H2O and O2 by a thermal catalytic process) has attracted extensive attention recently.1,2 Contrary to these reports, we found that not only the photocatalytic H2 generation could be driven by visible light but also the two-electron H2O2 disproportionation to form H2O and O2 could also be photocatalyzed by visible light over g-C3N4 catalysts. Photocatalytic H2, O2 generation, and simultaneous H2O2 formation in Cu/C3N4 and Fe/C3N4 dispersions were confirmed, about 2.1 and 1.4 μmol of H2 and 0.8 and 0.5 μmol of O2 evolved over Cu/C3N4 and Fe/C3N4 in 12 h, respectively. To prove the photocatalytic process of H2O2 disproportionation, the H2O2 was added as a reagent in g-C3N4, Cu/C3N4, and Fe/C3N4 dispersions. The results showed that the activity of H2 evolution decreased with the increase of H2O2 concentration; the corresponding AQEs of oxygen formation were 16.1%, 42.6%, and 78.5% at 400 nm, respectively. The remarkable increase of anodic photocurrents over Fe/C3N4/ITO and Cu/C3N4/ITO electrodes indicated that the two-electron H2O2 disproportionation was catalyzed via surface photocatalytic mechanism. The ESR results implied reaction occurred by O2–· radical path over g-C3N4 under irradiation.
中文翻译:

铜和铁掺杂的gC 3 N 4上的可见光催化水分解和光催化双电子氧形成
通过两个双电子过程分解水(H 2 O首先在可见光照射下光催化转化为H 2和H 2 O 2,然后通过热催化过程将H 2 O 2歧化为H 2 O和O 2)1,2与这些报道相反,我们发现不仅可见光可以驱动光催化H 2的生成,而且二电子H 2 O 2歧化形成H 2 O和O 2也可以。在gC上被可见光光催化3 N 4催化剂。光催化ħ 2,O- 2代,和同时ħ 2 ö 2形成在铜/ C 3 Ñ 4和Fe / C 3个Ñ 4分散体被证实,约2.1及H 1.4微摩尔2和0.8和0.5微摩尔的O- 2演变分别在12 h内在Cu / C 3 N 4和Fe / C 3 N 4上沉积。为了证明H 2 O 2歧化的光催化过程,H 2 O 2在试剂中以gC 3 N 4,Cu / C 3 N 4和Fe / C 3 N 4分散液的形式添加作为试剂。结果表明,H的活性2演化用H的增加而降低2 ö 2浓度; 在400 nm处,相应的氧生成AQE分别为16.1%,42.6%和78.5%。阳极光电流在Fe / C 3 N 4 / ITO和Cu / C 3 N 4 / ITO电极上的显着增加表明,双电子H 2 O 2歧化是通过表面光催化机理来催化的。ESR结果表明在辐照下,O 2 – ·自由基路径在gC 3 N 4上发生了反应。
更新日期:2015-12-22
中文翻译:

铜和铁掺杂的gC 3 N 4上的可见光催化水分解和光催化双电子氧形成
通过两个双电子过程分解水(H 2 O首先在可见光照射下光催化转化为H 2和H 2 O 2,然后通过热催化过程将H 2 O 2歧化为H 2 O和O 2)1,2与这些报道相反,我们发现不仅可见光可以驱动光催化H 2的生成,而且二电子H 2 O 2歧化形成H 2 O和O 2也可以。在gC上被可见光光催化3 N 4催化剂。光催化ħ 2,O- 2代,和同时ħ 2 ö 2形成在铜/ C 3 Ñ 4和Fe / C 3个Ñ 4分散体被证实,约2.1及H 1.4微摩尔2和0.8和0.5微摩尔的O- 2演变分别在12 h内在Cu / C 3 N 4和Fe / C 3 N 4上沉积。为了证明H 2 O 2歧化的光催化过程,H 2 O 2在试剂中以gC 3 N 4,Cu / C 3 N 4和Fe / C 3 N 4分散液的形式添加作为试剂。结果表明,H的活性2演化用H的增加而降低2 ö 2浓度; 在400 nm处,相应的氧生成AQE分别为16.1%,42.6%和78.5%。阳极光电流在Fe / C 3 N 4 / ITO和Cu / C 3 N 4 / ITO电极上的显着增加表明,双电子H 2 O 2歧化是通过表面光催化机理来催化的。ESR结果表明在辐照下,O 2 – ·自由基路径在gC 3 N 4上发生了反应。































 京公网安备 11010802027423号
京公网安备 11010802027423号