Nano Energy ( IF 16.8 ) Pub Date : 2018-06-14 , DOI: 10.1016/j.nanoen.2018.06.046 Jing Xu , Wenxue Zhang , Hongbo Fan , Faliang Cheng , Dawei Su , Guoxiu Wang
|
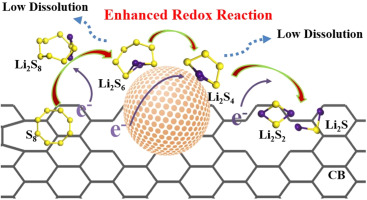
Entrapping polysulfide from dissolution into electrolyte by strong chemisorption of polar materials has been widely reported in lithium-sulfur (Li-S) battery. Here, for the first time, zinc sulfide (ZnS) was demonstrated as an activation catalyst in Li-S battery to suppress the soluble polysulfide shuttle effect by powering kinetics redox reactions of lithium polysulfide/sulfide. Kinetic analyses comprehensively identify that ZnS not only facilities polysulfide redox kinetics in liquid phase (Li2S8→Li2S6→Li2S4), but also promotes the effective decompositions of lithium sulfide (Li2S). Furthermore, first-principle calculations confirm that the low lithium ion diffusion barrier on the surface of ZnS promotes the redox reaction between lithium ion and sulfur species; and the low migration energy barrier of polysulfide on its surface guarantees the fast diffussion of polysulfides from the ZnS surface to the nearby conductive substrate, thus effectively smoothes polysulfides’ entrapping-diffusion-conversion mechanism across the ZnS interface, resulting in the highly reversible electrochemical performance. As evidenced by the ex situ SEM and visible experiment, the reaction between migrated sulfur species and lithium anode was significantly alleviated, and the insulating Li2S/Li2S2 was uniformly deposited on the ZnS-CB/S cathode. This ZnS cathode based Li-S battery exhibits outstanding performance including an excellent retained discharge specific capacity of 589 mA h gsul−1 with the high sulfur loading of 7 mg cm−2 (200 cycles) and extended cycling stability at the high current rate of 2 C, 5 C (632, 388 mA h gsul−1 after 1000 cycles).
中文翻译:

催化硫化锌促进高性能锂硫电池促进多硫化锂/硫化物的氧化还原动力学
在锂-硫(Li-S)电池中,已经广泛报道了通过极性材料的强化学吸附将多硫化物从电解质中截留的原因。在这里,首次展示了硫化锌(ZnS)作为Li-S电池中的活化催化剂,通过为多硫化锂/硫化锂的动力学氧化还原反应提供动力来抑制可溶性多硫化物的穿梭效应。动力学分析综合确定,ZnS不仅可以促进液相中的多硫化物氧化还原动力学(Li 2 S 8 →Li 2 S 6 →Li 2 S 4),而且还可以促进硫化锂(Li 2 S )的有效分解。S)。此外,第一性原理计算证实,ZnS表面的低锂离子扩散势垒促进了锂离子与硫物种之间的氧化还原反应。多硫化物在其表面上的低迁移能垒保证了多硫化物从ZnS表面到附近导电基底的快速扩散,从而有效地平滑了多硫化物在ZnS界面上的俘获-扩散-转化机制,从而实现了高度可逆的电化学性能。通过异位扫描电镜和可见光实验证明,迁移的硫物质与锂阳极之间的反应得到明显缓解,绝缘Li 2 S / Li 2 S 2均匀地沉积在ZnS-CB / S阴极上。这款基于ZnS阴极的Li-S电池具有出色的性能,包括589 mA h g sul -1的出色的保留放电比容量和7 mg cm -2的高硫负荷(200个循环),以及在高电流速率下的延长的循环稳定性。 2 C,5 C(1000个循环后的632,388 mA hg sul -1)。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号