当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multichannel Gas-Phase Unimolecular Decomposition of Acetone: Theoretical Kinetic Studies
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-06-13 00:00:00 , DOI: 10.1021/acs.jpca.8b02423 Vahid Saheb 1 , Meymanat Zokaie 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-06-13 00:00:00 , DOI: 10.1021/acs.jpca.8b02423 Vahid Saheb 1 , Meymanat Zokaie 1
Affiliation
|
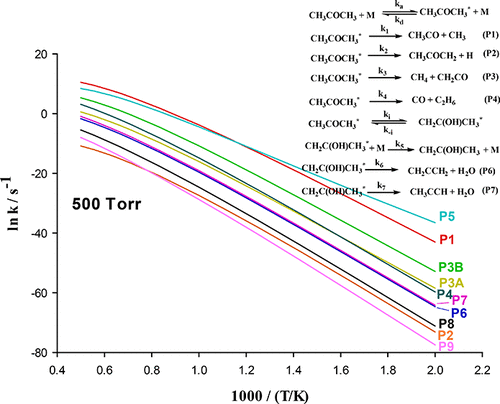
The multichannel thermal decomposition of acetone is studied theoretically. The isomerization of acetone molecule to its enol form, 1-propene-2-ol, is of especial interest in this research. Steady-state approximation is applied to the thermally activated species CH3COCH3* and CH2C(CH3)OH*, and by performing some statistical mechanical manipulations, integral expressions for the rate constants for the formation of different products are derived. The geometries of the reactant, intermediates, transition states, and products of the reaction are optimized at the MP2(full)/6-311++G(2d,2p) level of theory. More accurate energies are evaluated by single-point energy calculations at the CBS-Q, G4, and CCSD(T,full)/augh-cc-pVTZ+2df levels of theory. In order to account correctly for vibrational anharmonicities and tunneling effects, microcanonical rate constants for various channels are computed by using semiclassical transition state theory. It is found that the isomerization of CH3COCH3 to the enol form CH2C(CH3)OH plays an important role in the unimolecular decomposition reaction of CH3COCH3. The possible products originating from unimolecular decomposition of CH3COCH3 and CH2C(CH3)OH are investigated. It is revealed from present computed rate coefficients that the dominant product channel is the formation of CH2C(CH3)OH at low temperatures and high pressures due to the low barrier height for the isomerization process CH3COCH3 → CH2C(CH3)OH. However, at high temperatures and low pressures, the product channel CH3 + CH3CO becomes dominant. Also, the roaming product channels CH2CO + CH4 and C2H6 + CO could be important at high temperatures.
中文翻译:

丙酮的多通道气相单分子分解:理论动力学研究
从理论上研究了丙酮的多通道热分解。丙酮分子异构化为其烯醇形式1-丙烯-2-醇在本研究中特别受关注。对热活化物质CH 3 COCH 3 *和CH 2 C(CH 3OH *,并通过执行一些统计机械操作,得出用于形成不同产物的速率常数的积分表达式。在理论上的MP2(full)/ 6-311 ++ G(2d,2p)水平上优化了反应物的几何形状,中间体,过渡态和反应产物。通过在CBS-Q,G4和CCSD(T,full)/ augh-cc-pVTZ + 2df理论水平上的单点能量计算可以评估更准确的能量。为了正确考虑振动的非谐性和隧穿效应,使用半经典过渡态理论计算了各种通道的微规范速率常数。发现CH 3 COCH 3异构化为烯醇形式CH 2 C(CH 3)OH在CH 3 COCH 3的单分子分解反应中起重要作用。研究了可能由CH 3 COCH 3和CH 2 C(CH 3)OH的单分子分解产生的产物。它是从当前计算速率系数,发现该主导产品通道是CH的形成2 C(CH 3)OH在低温和高压下,由于异构化过程中的低势垒高度CH 3 COCH 3 →CH 2 C( CH 3)OH。但是,在高温和低压下,产品通道CH 3+ CH 3 CO占主导地位。同样,漫游产品通道CH 2 CO + CH 4和C 2 H 6 + CO在高温下可能很重要。
更新日期:2018-06-13
中文翻译:

丙酮的多通道气相单分子分解:理论动力学研究
从理论上研究了丙酮的多通道热分解。丙酮分子异构化为其烯醇形式1-丙烯-2-醇在本研究中特别受关注。对热活化物质CH 3 COCH 3 *和CH 2 C(CH 3OH *,并通过执行一些统计机械操作,得出用于形成不同产物的速率常数的积分表达式。在理论上的MP2(full)/ 6-311 ++ G(2d,2p)水平上优化了反应物的几何形状,中间体,过渡态和反应产物。通过在CBS-Q,G4和CCSD(T,full)/ augh-cc-pVTZ + 2df理论水平上的单点能量计算可以评估更准确的能量。为了正确考虑振动的非谐性和隧穿效应,使用半经典过渡态理论计算了各种通道的微规范速率常数。发现CH 3 COCH 3异构化为烯醇形式CH 2 C(CH 3)OH在CH 3 COCH 3的单分子分解反应中起重要作用。研究了可能由CH 3 COCH 3和CH 2 C(CH 3)OH的单分子分解产生的产物。它是从当前计算速率系数,发现该主导产品通道是CH的形成2 C(CH 3)OH在低温和高压下,由于异构化过程中的低势垒高度CH 3 COCH 3 →CH 2 C( CH 3)OH。但是,在高温和低压下,产品通道CH 3+ CH 3 CO占主导地位。同样,漫游产品通道CH 2 CO + CH 4和C 2 H 6 + CO在高温下可能很重要。





























 京公网安备 11010802027423号
京公网安备 11010802027423号