当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
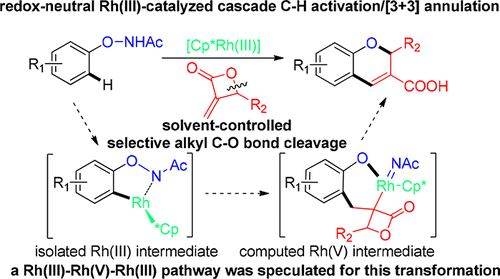
2H-Chromene-3-carboxylic Acid Synthesis via Solvent-Controlled and Rhodium(III)-Catalyzed Redox-Neutral C–H Activation/[3 + 3] Annulation Cascade
Organic Letters ( IF 4.9 ) Pub Date : 2018-06-13 00:00:00 , DOI: 10.1021/acs.orglett.8b01477 Zhi Zhou 1 , Mengyao Bian 1 , Lixin Zhao 1 , Hui Gao 1 , Junjun Huang 1 , Xiawen Liu 1 , Xiyong Yu 1 , Xingwei Li 2 , Wei Yi 1
Organic Letters ( IF 4.9 ) Pub Date : 2018-06-13 00:00:00 , DOI: 10.1021/acs.orglett.8b01477 Zhi Zhou 1 , Mengyao Bian 1 , Lixin Zhao 1 , Hui Gao 1 , Junjun Huang 1 , Xiawen Liu 1 , Xiyong Yu 1 , Xingwei Li 2 , Wei Yi 1
Affiliation
|
An efficient and redox-neutral synthesis of 2H-chromene-3-carboxylic acids from N-phenoxyacetamides and methyleneoxetanones has been realized via a solvent-controlled and rhodium(III)-catalyzed C–H activation/unusual [3 + 3] annulation sequence. This transformation represents the first example of using an α-methylene-β-lactone unit as the three-carbon source in transition-metal-catalyzed C–H activations through selective alkyl C–O bond cleavage. Synthetic applications and mechanistic details, including further derivatization of 2H-chromene-3-carboxylic acids, the isolation and identification of a five-membered rhodacycle, as well as the theoretical studies for reasoning a plausible Rh(III)–Rh(V)–Rh(III) process, have also been discussed.
中文翻译:

溶剂控制和铑(III)催化的氧化还原-中性CH活化/ [3 + 3]级联反应合成2 H-铬-3-羧酸
通过溶剂控制和铑(III)催化的C–H活化/不寻常的[3 + 3]环化反应,已经实现了由N-苯氧基乙酰胺和亚甲基氧杂环丁烷高效合成和氧化还原中性的2 H-苯甲基-3-羧酸顺序。这种转变代表了使用α-亚甲基-β-内酯单元作为过渡碳催化的CH活化(通过选择性烷基C-O键断裂)中的三碳源的第一个例子。合成应用和机理细节,包括2 H-苯并-3-羧酸的进一步衍生化,五元罗丹环的分离和鉴定以及合理的Rh(III)-Rh(V)的理论研究-Rh(III)过程,也已经讨论过。
更新日期:2018-06-13
中文翻译:

溶剂控制和铑(III)催化的氧化还原-中性CH活化/ [3 + 3]级联反应合成2 H-铬-3-羧酸
通过溶剂控制和铑(III)催化的C–H活化/不寻常的[3 + 3]环化反应,已经实现了由N-苯氧基乙酰胺和亚甲基氧杂环丁烷高效合成和氧化还原中性的2 H-苯甲基-3-羧酸顺序。这种转变代表了使用α-亚甲基-β-内酯单元作为过渡碳催化的CH活化(通过选择性烷基C-O键断裂)中的三碳源的第一个例子。合成应用和机理细节,包括2 H-苯并-3-羧酸的进一步衍生化,五元罗丹环的分离和鉴定以及合理的Rh(III)-Rh(V)的理论研究-Rh(III)过程,也已经讨论过。







































 京公网安备 11010802027423号
京公网安备 11010802027423号