当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel-Catalyzed Denitrogenative Cross-Coupling Reaction of 1,2,3-Benzotriazin-4(3H)-ones with Organoboronic Acids: An Easy Access to Ortho-Arylated and Alkenylated Benzamides
Organic Letters ( IF 4.9 ) Pub Date : 2018-06-13 00:00:00 , DOI: 10.1021/acs.orglett.8b01401 Madasamy Hari Balakrishnan 1 , Kotturaja Sathriyan 1 , Subramaniyan Mannathan 1, 2
Organic Letters ( IF 4.9 ) Pub Date : 2018-06-13 00:00:00 , DOI: 10.1021/acs.orglett.8b01401 Madasamy Hari Balakrishnan 1 , Kotturaja Sathriyan 1 , Subramaniyan Mannathan 1, 2
Affiliation
|
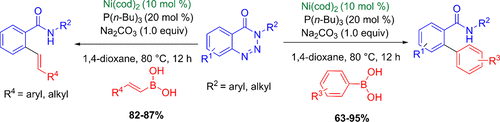
A novel nickel-catalyzed approach to synthesize ortho-arylated and alkenylated benzamides in good to high yields via a denitrogenative cross-coupling reaction of 1,2,3-benzotriazin-4(3H)-ones with organoboronic acids is described. The reaction proceeds through a five-membered azanickelacyclic intermediate with the extrusion of a nitrogen molecule. Moreover, the resulting ortho-arylated benzamides were successfully converted into synthetically useful substituted fluorenones and ortho-arylated benzylamine derivatives in high yields.
中文翻译:

1,2,3-苯并三嗪-4(3 H)-酮与有机硼酸的镍催化脱氮交叉偶联反应:轻松获得邻位-芳基化和烯化的苯甲酰胺
一种新的镍催化的方法来合成邻-arylated和经由1,2,3-苯并三嗪-4(3的denitrogenative交叉偶联反应以优良至高产率链烯基苯甲酰胺ħ) -酮与有机硼酸进行说明。该反应通过五元氮杂氮杂环中间体与氮分子的挤出而进行。此外,将得到的邻芳基化的苯甲酰胺成功地以高收率转化成合成上有用的取代的芴酮和邻芳基化的苄胺衍生物。
更新日期:2018-06-13
中文翻译:

1,2,3-苯并三嗪-4(3 H)-酮与有机硼酸的镍催化脱氮交叉偶联反应:轻松获得邻位-芳基化和烯化的苯甲酰胺
一种新的镍催化的方法来合成邻-arylated和经由1,2,3-苯并三嗪-4(3的denitrogenative交叉偶联反应以优良至高产率链烯基苯甲酰胺ħ) -酮与有机硼酸进行说明。该反应通过五元氮杂氮杂环中间体与氮分子的挤出而进行。此外,将得到的邻芳基化的苯甲酰胺成功地以高收率转化成合成上有用的取代的芴酮和邻芳基化的苄胺衍生物。






























 京公网安备 11010802027423号
京公网安备 11010802027423号