当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Sterol 14α-Demethylase Structure-Based Design of VNI ((R)-N-(1-(2,4-Dichlorophenyl)-2-(1H-imidazol-1-yl)ethyl)-4-(5-phenyl-1,3,4-oxadiazol-2-yl)benzamide)) Derivatives To Target Fungal Infections: Synthesis, Biological Evaluation, and Crystallographic Analysis
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-06-12 00:00:00 , DOI: 10.1021/acs.jmedchem.8b00641 Laura Friggeri 1 , Tatiana Y. Hargrove 1 , Zdzislaw Wawrzak 2 , Anna L. Blobaum 3 , Girish Rachakonda 4 , Craig W. Lindsley 3 , Fernando Villalta 4 , W. David Nes 5 , Maurizio Botta 6 , F. Peter Guengerich 1 , Galina I. Lepesheva 1, 7
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-06-12 00:00:00 , DOI: 10.1021/acs.jmedchem.8b00641 Laura Friggeri 1 , Tatiana Y. Hargrove 1 , Zdzislaw Wawrzak 2 , Anna L. Blobaum 3 , Girish Rachakonda 4 , Craig W. Lindsley 3 , Fernando Villalta 4 , W. David Nes 5 , Maurizio Botta 6 , F. Peter Guengerich 1 , Galina I. Lepesheva 1, 7
Affiliation
|
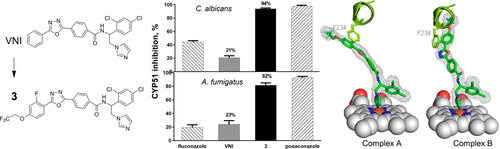
Because of the increase in the number of immunocompromised patients, the incidence of invasive fungal infections is growing, but the treatment efficiency remains unacceptably low. The most potent clinical systemic antifungals (azoles) are the derivatives of two scaffolds: ketoconazole and fluconazole. Being the safest antifungal drugs, they still have shortcomings, mainly because of pharmacokinetics and resistance. Here, we report the successful use of the target fungal enzyme, sterol 14α-demethylase (CYP51), for structure-based design of novel antifungal drug candidates by minor modifications of VNI [(R)-N-(1-(2,4-dichlorophenyl)-2-(1H-imidazol-1-yl)ethyl)-4-(5-phenyl-1,3,4-oxadiazol-2-yl)benzamide)], an inhibitor of protozoan CYP51 that cures Chagas disease. The synthesis of fungi-oriented VNI derivatives, analysis of their potencies to inhibit CYP51s from two major fungal pathogens (Aspergillus fumigatus and Candida albicans), microsomal stability, effects in fungal cells, and structural characterization of A. fumigatus CYP51 in complexes with the most potent compound are described, offering a new antifungal drug scaffold and outlining directions for its further optimization.
中文翻译:

基于甾醇14α-脱甲基酶结构的VNI((R)-N-(1-(2,4-二氯苯基)-2-(1 H-咪唑-1-基)乙基)-4-(5-苯基- 1,3,4-恶二唑-2-基)苯甲酰胺))靶向真菌感染的衍生物:合成,生物学评估和晶体学分析
由于免疫受损患者数量的增加,侵袭性真菌感染的发生率正在增加,但是治疗效率仍然低得令人无法接受。临床上最有效的系统性抗真菌药(唑类)是两种支架的衍生物:酮康唑和氟康唑。作为最安全的抗真菌药物,它们仍然存在缺点,主要是由于药代动力学和耐药性。在这里,我们报告了目标真菌酶,甾醇14α-脱甲基酶(CYP51),通过对VNI [(R)-N-(1-(2,4, -二氯苯基)-2-(1 H-咪达唑-1-基)乙基)-4-(5-苯基-1,3,4-恶二唑-2-基)苯甲酰胺)],这是一种原生动物CYP51的抑制剂,可治愈南美锥虫病。面向真菌的VNI衍生物的合成,其抑制两种主要真菌病原体(烟曲霉和白色念珠菌)CYP51s的能力,微粒体稳定性,对真菌细胞的作用以及最复杂的烟曲霉CYP51的结构表征描述了有效的化合物,提供了新的抗真菌药物支架并概述了进一步优化的方向。
更新日期:2018-06-12
中文翻译:

基于甾醇14α-脱甲基酶结构的VNI((R)-N-(1-(2,4-二氯苯基)-2-(1 H-咪唑-1-基)乙基)-4-(5-苯基- 1,3,4-恶二唑-2-基)苯甲酰胺))靶向真菌感染的衍生物:合成,生物学评估和晶体学分析
由于免疫受损患者数量的增加,侵袭性真菌感染的发生率正在增加,但是治疗效率仍然低得令人无法接受。临床上最有效的系统性抗真菌药(唑类)是两种支架的衍生物:酮康唑和氟康唑。作为最安全的抗真菌药物,它们仍然存在缺点,主要是由于药代动力学和耐药性。在这里,我们报告了目标真菌酶,甾醇14α-脱甲基酶(CYP51),通过对VNI [(R)-N-(1-(2,4, -二氯苯基)-2-(1 H-咪达唑-1-基)乙基)-4-(5-苯基-1,3,4-恶二唑-2-基)苯甲酰胺)],这是一种原生动物CYP51的抑制剂,可治愈南美锥虫病。面向真菌的VNI衍生物的合成,其抑制两种主要真菌病原体(烟曲霉和白色念珠菌)CYP51s的能力,微粒体稳定性,对真菌细胞的作用以及最复杂的烟曲霉CYP51的结构表征描述了有效的化合物,提供了新的抗真菌药物支架并概述了进一步优化的方向。







































 京公网安备 11010802027423号
京公网安备 11010802027423号