当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Human Amyloid Precursor Protein Binds Copper Ions Dominated by a Picomolar-Affinity Site in the Helix-Rich E2 Domain
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-12 00:00:00 , DOI: 10.1021/acs.biochem.8b00572 Tessa R. Young 1 , Tara L. Pukala 2 , Roberto Cappai 3 , Anthony G. Wedd 1 , Zhiguang Xiao 1, 4
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-12 00:00:00 , DOI: 10.1021/acs.biochem.8b00572 Tessa R. Young 1 , Tara L. Pukala 2 , Roberto Cappai 3 , Anthony G. Wedd 1 , Zhiguang Xiao 1, 4
Affiliation
|
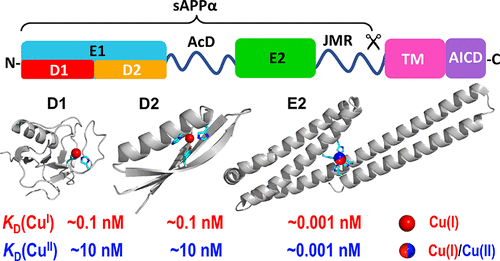
A manifestation of Alzheimer’s disease (AD) is the aggregation in the brain of amyloid β (Aβ) peptides derived from the amyloid precursor protein (APP). APP has been linked to modulation of normal copper homeostasis, while dysregulation of Aβ production and clearance has been associated with disruption of copper balance. However, quantitative copper chemistry on APP is lacking, in contrast to the plethora of copper chemistry available for Aβ peptides. The soluble extracellular protein domain sAPPα (molar mass including post-translational modifications of ∼100 kDa) has now been isolated in good yield and high quality. It is known to feature several copper binding sites with different affinities. However, under Cu-limiting conditions, it binds either Cu(I) or Cu(II) with picomolar affinity at a single site (labeled M1) that is located within the APP E2 subdomain. M1 in E2 was identified previously by X-ray crystallography as a Cu(II) site that features four histidine side chains (H313, H386, H432, and H436) as ligands. The presence of CuII(His)4 is confirmed in solution at pH ≤7.4, while Cu(I) binding involves either the same ligands or a subset. The binding affinities are pH-dependent, and the picomolar affinities for both Cu(I) and Cu(II) at pH 7.4 indicate that either oxidation state may be accessible under physiological conditions. Redox activity was observed in the presence of an electron donor (ascorbate) and acceptor (dioxygen). A critical analysis of the potential biological implications of these findings is presented.
中文翻译:

人类淀粉样蛋白前体蛋白结合富含螺旋结构的E2域中以皮摩尔亲和力位点为主的铜离子
阿尔茨海默氏病(AD)的一种表现是源自淀粉样前体蛋白(APP)的淀粉样β(Aβ)肽在大脑中的聚集。APP与正常铜稳态的调节有关,而Aβ产生和清除的失调与铜平衡的破坏有关。然而,与可用于Aβ肽的大量铜化学相反,APP上缺乏定量铜化学。现已分离出可溶的细胞外蛋白结构域sAPPα(摩尔质量,包括约100 kDa的翻译后修饰),收率高,质量高。已知具有多个具有不同亲和力的铜结合位点。但是,在铜限制条件下,它在APP E2子域内的单个位点(标记为M1)上以皮摩尔亲和力结合Cu(I)或Cu(II)。E2中的M1先前已通过X射线晶体学鉴定为一个以四个组氨酸侧链(H313,H386,H432和H436)为配体的Cu(II)位点。铜的存在II(His)4在pH≤7.4的溶液中得到确认,而Cu(I)结合涉及相同的配体或子集。结合亲和力是pH依赖性的,在pH 7.4时对Cu(I)和Cu(II)的皮摩尔亲和力表明,在生理条件下,任何一种氧化态都可以达到。在电子给体(抗坏血酸)和受体(双氧)的存在下观察到氧化还原活性。提出了对这些发现的潜在生物学意义的批判性分析。
更新日期:2018-06-12
中文翻译:

人类淀粉样蛋白前体蛋白结合富含螺旋结构的E2域中以皮摩尔亲和力位点为主的铜离子
阿尔茨海默氏病(AD)的一种表现是源自淀粉样前体蛋白(APP)的淀粉样β(Aβ)肽在大脑中的聚集。APP与正常铜稳态的调节有关,而Aβ产生和清除的失调与铜平衡的破坏有关。然而,与可用于Aβ肽的大量铜化学相反,APP上缺乏定量铜化学。现已分离出可溶的细胞外蛋白结构域sAPPα(摩尔质量,包括约100 kDa的翻译后修饰),收率高,质量高。已知具有多个具有不同亲和力的铜结合位点。但是,在铜限制条件下,它在APP E2子域内的单个位点(标记为M1)上以皮摩尔亲和力结合Cu(I)或Cu(II)。E2中的M1先前已通过X射线晶体学鉴定为一个以四个组氨酸侧链(H313,H386,H432和H436)为配体的Cu(II)位点。铜的存在II(His)4在pH≤7.4的溶液中得到确认,而Cu(I)结合涉及相同的配体或子集。结合亲和力是pH依赖性的,在pH 7.4时对Cu(I)和Cu(II)的皮摩尔亲和力表明,在生理条件下,任何一种氧化态都可以达到。在电子给体(抗坏血酸)和受体(双氧)的存在下观察到氧化还原活性。提出了对这些发现的潜在生物学意义的批判性分析。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号