Nature Chemistry ( IF 19.2 ) Pub Date : 2015-12-21 , DOI: 10.1038/nchem.2414
William M. Aumiller , Christine D. Keating
|
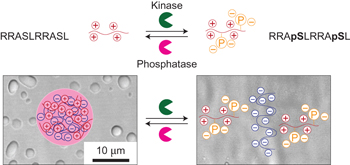
Biological cells are highly organized, with numerous subcellular compartments. Phosphorylation has been hypothesized as a means to control the assembly/disassembly of liquid-like RNA- and protein-rich intracellular bodies, or liquid organelles, that lack delimiting membranes. Here, we demonstrate that charge-mediated phase separation, or complex coacervation, of RNAs with cationic peptides can generate simple model liquid organelles capable of reversibly compartmentalizing biomolecules. Formation and dissolution of these liquid bodies was controlled by changes in peptide phosphorylation state using a kinase/phosphatase enzyme pair. The droplet-generating phase transition responded to modification of even a single serine residue. Electrostatic interactions between the short cationic peptides and the much longer polyanionic RNAs drove phase separation. Coacervates were also formed on silica beads, a primitive model for localization at specific intracellular sites. This work supports phosphoregulation of complex coacervation as a viable mechanism for dynamic intracellular compartmentalization in membraneless organelles.
中文翻译:

磷酸化介导的RNA /肽复合物凝聚作为细胞内液体细胞器的模型
生物细胞是高度组织的,具有许多亚细胞区室。磷酸化被认为是控制缺乏定膜的富含液体和液体的富含RNA和蛋白质的细胞内体或液体细胞器的组装/拆卸的一种手段。在这里,我们证明了带阳离子肽的RNA的电荷介导的相分离或复杂凝聚,可以产生能够可逆地分隔生物分子的简单模型液体细胞器。这些液体的形成和溶解是通过使用激酶/磷酸酶对改变肽的磷酸化状态来控制的。产生液滴的相变甚至响应单个丝氨酸残基的修饰。短阳离子肽和长得多的聚阴离子RNA之间的静电相互作用推动了相分离。凝聚层也形成在二氧化硅珠上,二氧化硅珠是定位在特定细胞内位点的原始模型。这项工作支持复杂凝聚的磷酸化作为无膜细胞器中动态细胞内区室化的可行机制。

































 京公网安备 11010802027423号
京公网安备 11010802027423号