当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A New Class of Orthosteric uPAR·uPA Small-Molecule Antagonists Are Allosteric Inhibitors of the uPAR·Vitronectin Interaction
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2015-03-31 00:00:00 , DOI: 10.1021/cb500832q Degang Liu , Donghui Zhou , Bo Wang 1 , William Eric Knabe , Samy O. Meroueh 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2015-03-31 00:00:00 , DOI: 10.1021/cb500832q Degang Liu , Donghui Zhou , Bo Wang 1 , William Eric Knabe , Samy O. Meroueh 1
Affiliation
|
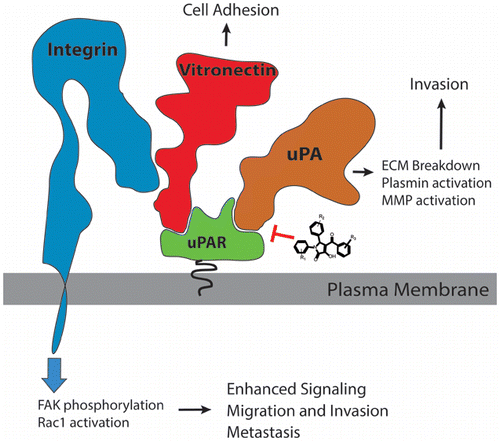
The urokinase receptor (uPAR) is a GPI-anchored cell surface receptor that is at the center of an intricate network of protein–protein interactions. Its immediate binding partners are the serine proteinase urokinase (uPA), and vitronectin (VTN), a component of the extracellular matrix. uPA and VTN bind at distinct sites on uPAR to promote extracellular matrix degradation and integrin signaling, respectively. Here, we report the discovery of a new class of pyrrolone small-molecule inhibitors of the tight ∼1 nM uPAR·uPA protein–protein interaction. These compounds were designed to bind to the uPA pocket on uPAR. The highest affinity compound, namely 7, displaced a fluorescently labeled α-helical peptide (AE147-FAM) with an inhibition constant Ki of 0.7 μM and inhibited the tight uPAR·uPAATF interaction with an IC50 of 18 μM. Biophysical studies with surface plasmon resonance showed that VTN binding is highly dependent on uPA. This cooperative binding was confirmed as 7, which binds at the uPAR·uPA interface, also inhibited the distal VTN·uPAR interaction. In cell culture, 7 blocked the uPAR·uPA interaction in uPAR-expressing human embryonic kidney (HEK-293) cells and impaired cell adhesion to VTN, a process that is mediated by integrins. As a result, 7 inhibited integrin signaling in MDA-MB-231 cancer cells as evidenced by a decrease in focal adhesion kinase (FAK) phosphorylation and Rac1 GTPase activation. Consistent with these results, 7 blocked breast MDA-MB-231 cancer cell invasion with IC50 values similar to those observed in ELISA and surface plasmon resonance competition studies. Explicit-solvent molecular dynamics simulations show that the cooperativity between uPA and VTN is attributed to stabilization of uPAR motion by uPA. In addition, free energy calculations revealed that uPA stabilizes the VTNSMB·uPAR interaction through more favorable electrostatics and entropy. Disruption of the uPAR·VTNSMB interaction by 7 is consistent with the cooperative binding to uPAR by uPA and VTN. Interestingly, the VTNSMB·uPAR interaction was less favorable in the VTNSMB·uPAR·7 complex suggesting potential cooperativity between 7 and VTN. Compound 7 provides an excellent starting point for the development of more potent derivatives to explore uPAR biology.
中文翻译:

一类新的正构uPAR·uPA小分子拮抗剂是uPAR·玻连蛋白相互作用的变构抑制剂。
尿激酶受体(uPAR)是GPI锚定的细胞表面受体,位于复杂的蛋白质与蛋白质相互作用网络的中心。它的直接结合伙伴是丝氨酸蛋白酶尿激酶(uPA)和玻连蛋白(VTN),它们是细胞外基质的组成部分。uPA和VTN分别结合在uPAR的不同位点上,以促进细胞外基质降解和整联蛋白信号传导。在这里,我们报告发现了一种新型的〜1 nM uPAR·uPA蛋白质与蛋白质相互作用紧密的吡咯烷酮小分子抑制剂。这些化合物被设计为与uPAR的uPA口袋结合。亲和性最高的化合物7取代了具有抑制常数K i的荧光标记的α-螺旋肽(AE147-FAM)浓度为0.7μM,并抑制了uPAR·uPA ATF的紧密相互作用,IC 50为18μM。具有表面等离子体共振的生物物理研究表明,VTN结合高度依赖uPA。确认该协同结合为7,其在uPAR·uPA界面处结合,也抑制了远端VTN·uPAR相互作用。在细胞培养中,有7种物质阻断了表达uPAR的人胚肾(HEK-293)细胞中的uPAR·uPA相互作用,并损害了细胞与VTN的粘附,该过程由整联蛋白介导。结果,通过抑制粘着斑激酶(FAK)磷酸化和Rac1 GTPase活化,证明了7种抑制了MDA-MB-231癌细胞中整合素的信号传导。与这些结果一致,7阻断了乳腺癌MDA-MB-231癌细胞的侵袭,其IC 50值类似于ELISA和表面等离子体共振竞争研究中观察到的值。显式溶剂分子动力学模拟表明,uPA和VTN之间的协同作用归因于uPA稳定了uPAR运动。此外,自由能计算表明,uPA通过更有利的静电和熵来稳定VTN SMB ·uPAR相互作用。uPAR·VTN SMB交互的中断为7与uPA和VTN对uPAR的协作绑定一致。有趣的是,VTN SMB ·uPAR· 7中的VTN SMB ·uPAR交互作用较差。复杂,表明7和VTN之间可能存在合作性。化合物7为开发更强的衍生物以探索uPAR生物学提供了一个极好的起点。
更新日期:2015-03-31
中文翻译:

一类新的正构uPAR·uPA小分子拮抗剂是uPAR·玻连蛋白相互作用的变构抑制剂。
尿激酶受体(uPAR)是GPI锚定的细胞表面受体,位于复杂的蛋白质与蛋白质相互作用网络的中心。它的直接结合伙伴是丝氨酸蛋白酶尿激酶(uPA)和玻连蛋白(VTN),它们是细胞外基质的组成部分。uPA和VTN分别结合在uPAR的不同位点上,以促进细胞外基质降解和整联蛋白信号传导。在这里,我们报告发现了一种新型的〜1 nM uPAR·uPA蛋白质与蛋白质相互作用紧密的吡咯烷酮小分子抑制剂。这些化合物被设计为与uPAR的uPA口袋结合。亲和性最高的化合物7取代了具有抑制常数K i的荧光标记的α-螺旋肽(AE147-FAM)浓度为0.7μM,并抑制了uPAR·uPA ATF的紧密相互作用,IC 50为18μM。具有表面等离子体共振的生物物理研究表明,VTN结合高度依赖uPA。确认该协同结合为7,其在uPAR·uPA界面处结合,也抑制了远端VTN·uPAR相互作用。在细胞培养中,有7种物质阻断了表达uPAR的人胚肾(HEK-293)细胞中的uPAR·uPA相互作用,并损害了细胞与VTN的粘附,该过程由整联蛋白介导。结果,通过抑制粘着斑激酶(FAK)磷酸化和Rac1 GTPase活化,证明了7种抑制了MDA-MB-231癌细胞中整合素的信号传导。与这些结果一致,7阻断了乳腺癌MDA-MB-231癌细胞的侵袭,其IC 50值类似于ELISA和表面等离子体共振竞争研究中观察到的值。显式溶剂分子动力学模拟表明,uPA和VTN之间的协同作用归因于uPA稳定了uPAR运动。此外,自由能计算表明,uPA通过更有利的静电和熵来稳定VTN SMB ·uPAR相互作用。uPAR·VTN SMB交互的中断为7与uPA和VTN对uPAR的协作绑定一致。有趣的是,VTN SMB ·uPAR· 7中的VTN SMB ·uPAR交互作用较差。复杂,表明7和VTN之间可能存在合作性。化合物7为开发更强的衍生物以探索uPAR生物学提供了一个极好的起点。































 京公网安备 11010802027423号
京公网安备 11010802027423号