Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2018-06-08 , DOI: 10.1016/j.bioorg.2018.06.016 Neelesh Maheshwari , Chandrabose Karthikeyan , Shraddha V. Bhadada , Chandan Sahi , Amit K. Verma , N.S. Hari Narayana Moorthy , Piyush Trivedi
|
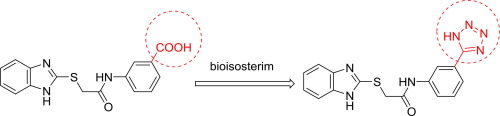
Described herein is the synthesis and biological evaluation of a series of non-carboxylic inhibitors of Protein Tyrosine Phosphatase 1B designed using bioisosteric replacement strategy. Six N-(3-(1H-tetrazol-5-yl)phenyl)acetamide derivatives designed employing the aforementioned strategy were synthesized and screened for PTP1B inhibitory activity. Among the synthesized compounds, compound NM-03 exhibited the most potent inhibitory activity with IC50 value of 4.48 µM. Docking studies with NM-03 revealed the key interactions with desired amino acids in the binding site of PTP1B. Furthermore, compound NM-03 also elicited good in vivo activity. Taken together, the results of this study establish N-(3-(1H-tetrazole-5-yl)phenyl)-2-(benzo[d]oxazol-2-ylthio)acetamide (NM-03) as a valuable lead molecule with great potential for PTP1B inhibitor development targeting diabetes.
中文翻译:

通过生物立体调节设计的一些N-(3-(1 H-四唑-5-基)苯基)乙酰胺衍生物作为新型非羧基PTP1B抑制剂的合成和生物学评价
本文描述了使用生物等排代换策略设计的一系列蛋白质酪氨酸磷酸酶1B的非羧酸类抑制剂的合成和生物学评估。合成了六种采用上述策略设计的N-(3-(1 H-四唑-5-基)苯基)乙酰胺衍生物,并筛选了PTP1B抑制活性。在合成的化合物中,化合物NM-03表现出最强的抑制活性,IC 50值为4.48 µM。与NM-03的对接研究揭示了与PTP1B结合位点中所需氨基酸的关键相互作用。此外,化合物NM-03在体内也具有良好的诱导作用活动。两者合计,这项研究的结果建立N-(3-(1 H-四唑-5-基)苯基)-2-(苯并[ d ]恶唑-2-基硫基)乙酰胺(NM-03)作为有价值的铅具有针对糖尿病的PTP1B抑制剂开发潜力的分子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号