Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Theoretical insights into the reaction mechanisms between 2,3,7,8-tetrachlorodibenzofuran and the methylidyne radical†
RSC Advances ( IF 3.9 ) Pub Date : 2018-06-08 00:00:00 , DOI: 10.1039/c8ra03046d Wenjing Wei 1 , Weihua Wang 1 , Kaining Xu 1 , Wenling Feng 1 , Xiaoping Li 1 , Ping Li 1
RSC Advances ( IF 3.9 ) Pub Date : 2018-06-08 00:00:00 , DOI: 10.1039/c8ra03046d Wenjing Wei 1 , Weihua Wang 1 , Kaining Xu 1 , Wenling Feng 1 , Xiaoping Li 1 , Ping Li 1
Affiliation
|
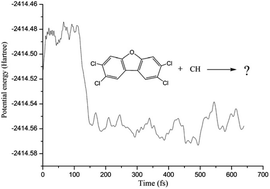
To explore the potential role of the methylidyne radical (CH) in the transformation of 2,3,7,8-tetrachlorodibenzofuran (TCDF), in this study, the detailed reaction mechanisms between TCDF and CH radical have been systematically investigated employing the B3LYP method of density functional theory (DFT) in combination with the atoms in molecules (AIM) theory and ab initio molecular dynamics. It was found that the title reaction is a multi-channel reaction, i.e., the CH radical can attack the C–X (X = C, Cl, H, O) bonds of TCDF via the insertion modes, resulting in the formation of 13 products. Thermodynamically, the whole reaction processes are exothermic and spontaneous since all the enthalpy and Gibbs free energy changes are negative values in the formation processes. Moreover, the thermodynamic stability of the products is controlled by the distribution of the single unpaired electron. Kinetically, the most favorable reaction channel is the insertion of the CH radical into the C–C bond except for the C atoms attached to the chlorine atom. Moreover, the dominant products have been further confirmed by the molecular dynamics. Meanwhile, the IR spectra and hyperfine coupling constants of the dominant products have been investigated to provide helpful information for their identification experimentally. In addition, the reactivity of the CH radical toward the F- and Br-substituted TCDFs has also been investigated. Expectedly, the present findings can enable us to better understand the reactivity of the CH radical toward organic pollutants analogous to TCDF in the atmosphere.
中文翻译:

2,3,7,8-四氯二苯并呋喃与亚甲基自由基之间反应机理的理论见解†
为了探索亚甲基自由基 (CH) 在 2,3,7,8-四氯二苯并呋喃 (TCDF) 转化中的潜在作用,本研究采用 B3LYP 方法系统研究了 TCDF 与 CH 自由基之间的详细反应机制密度泛函理论 (DFT) 与分子中的原子 (AIM) 理论和从头算分子动力学相结合。发现标题反应是一个多通道反应,即CH自由基可以通过攻击TCDF的C-X(X = C,Cl,H,O)键。插入模式,形成13个产品。在热力学上,整个反应过程是放热和自发的,因为所有的焓和吉布斯自由能变化在形成过程中都是负值。此外,产物的热力学稳定性受单个不成对电子的分布控制。动力学上,最有利的反应通道是将 CH 自由基插入到 C-C 键中,除了与氯原子相连的 C 原子。此外,优势产物已被分子动力学进一步证实。同时,研究了主要产物的红外光谱和超精细耦合常数,为实验鉴定提供了有用的信息。此外,还研究了 CH 自由基对 F 和 Br 取代的 TCDF 的反应性。预计,目前的研究结果可以使我们更好地了解 CH 自由基对大气中类似于 TCDF 的有机污染物的反应性。
更新日期:2018-06-08
中文翻译:

2,3,7,8-四氯二苯并呋喃与亚甲基自由基之间反应机理的理论见解†
为了探索亚甲基自由基 (CH) 在 2,3,7,8-四氯二苯并呋喃 (TCDF) 转化中的潜在作用,本研究采用 B3LYP 方法系统研究了 TCDF 与 CH 自由基之间的详细反应机制密度泛函理论 (DFT) 与分子中的原子 (AIM) 理论和从头算分子动力学相结合。发现标题反应是一个多通道反应,即CH自由基可以通过攻击TCDF的C-X(X = C,Cl,H,O)键。插入模式,形成13个产品。在热力学上,整个反应过程是放热和自发的,因为所有的焓和吉布斯自由能变化在形成过程中都是负值。此外,产物的热力学稳定性受单个不成对电子的分布控制。动力学上,最有利的反应通道是将 CH 自由基插入到 C-C 键中,除了与氯原子相连的 C 原子。此外,优势产物已被分子动力学进一步证实。同时,研究了主要产物的红外光谱和超精细耦合常数,为实验鉴定提供了有用的信息。此外,还研究了 CH 自由基对 F 和 Br 取代的 TCDF 的反应性。预计,目前的研究结果可以使我们更好地了解 CH 自由基对大气中类似于 TCDF 的有机污染物的反应性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号