当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Boronic Acid-Catalyzed, Highly Enantioselective Aza-Michael Additions of Hydroxamic Acid to Quinone Imine Ketals
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2015-12-17 , DOI: 10.1021/jacs.5b11518 Takuya Hashimoto 1 , Alberto Osuna Gálvez 1 , Keiji Maruoka 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2015-12-17 , DOI: 10.1021/jacs.5b11518 Takuya Hashimoto 1 , Alberto Osuna Gálvez 1 , Keiji Maruoka 1
Affiliation
|
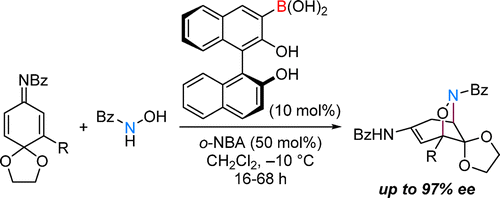
Boronic acid is one of the most versatile organic molecules in chemistry. Its uses include organic reactions, molecular recognition, assembly, and even medicine. While boronic acid catalysis, which utilizes an inherent catalytic property, has become an important research objective, it still lags far behind other boronic acid chemistries. Here, we report our discovery of a new boronic acid catalysis that enables the aza-Michael addition of hydroxamic acid to quinone imine ketals. By using 3-borono-BINOL as a chiral boronic acid catalyst, this reaction could be implemented in a highly enantioselective manner, paving the way to densely functionalized cyclohexanes.
中文翻译:

硼酸催化的高对映选择性氮杂-迈克尔加成异羟肟酸到醌亚胺缩酮
硼酸是化学中用途最广泛的有机分子之一。它的用途包括有机反应、分子识别、组装,甚至医学。虽然利用固有催化特性的硼酸催化已成为重要的研究目标,但它仍然远远落后于其他硼酸化学。在这里,我们报告了我们发现了一种新的硼酸催化剂,该催化剂能够将异羟肟酸与醌亚胺缩酮进行氮杂-迈克尔加成。通过使用 3-borono-BINOL 作为手性硼酸催化剂,该反应可以以高度对映选择性的方式实施,为密集官能化的环己烷铺平了道路。
更新日期:2015-12-17
中文翻译:

硼酸催化的高对映选择性氮杂-迈克尔加成异羟肟酸到醌亚胺缩酮
硼酸是化学中用途最广泛的有机分子之一。它的用途包括有机反应、分子识别、组装,甚至医学。虽然利用固有催化特性的硼酸催化已成为重要的研究目标,但它仍然远远落后于其他硼酸化学。在这里,我们报告了我们发现了一种新的硼酸催化剂,该催化剂能够将异羟肟酸与醌亚胺缩酮进行氮杂-迈克尔加成。通过使用 3-borono-BINOL 作为手性硼酸催化剂,该反应可以以高度对映选择性的方式实施,为密集官能化的环己烷铺平了道路。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号