当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Oxidation of α-trifluoromethyl and non-fluorinated alcohols via the merger of oxoammonium cations and photoredox catalysis†
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-06-05 00:00:00 , DOI: 10.1039/c8ob01063c Vincent A. Pistritto 1, 2, 3, 4 , Joshua M. Paolillo 1, 2, 3, 4 , Kathryn A. Bisset 1, 2, 3, 4 , Nicholas E. Leadbeater 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-06-05 00:00:00 , DOI: 10.1039/c8ob01063c Vincent A. Pistritto 1, 2, 3, 4 , Joshua M. Paolillo 1, 2, 3, 4 , Kathryn A. Bisset 1, 2, 3, 4 , Nicholas E. Leadbeater 1, 2, 3, 4
Affiliation
|
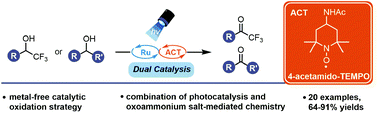
We present an alcohol oxidation strategy to access α-trifluoromethyl ketones (TFMKs) merging catalytic oxoammonium cation oxidation with visible-light photoredox catalysis. This work uses 4-acetamido-(2,2,6,6-tetramethyl-piperidin-1-yl)oxyl as an organic oxidant capable of generating TFMKs in good yields. The methodology serves as an improvement over previous reports of an analogous oxidation strategy requiring superstoichiometric quantities of oxidant. Both primary and secondary non-fluorinated alcohols can also be oxidised in good yields.
中文翻译:

通过氧铵阳离子的结合和光氧化还原催化 对α-三氟甲基和非氟化醇的氧化†
我们提出了一种醇氧化策略,以访问α-三氟甲基酮(TFMKs)合并可见光氧化还原催化的催化氧代铵阳离子氧化。这项工作使用4-乙酰氨基-(2,2,6,6-四甲基-哌啶-1-基)氧基作为有机氧化剂,能够以高收率产生TFMK。该方法是对先前报道的需要超化学计量量的氧化剂的类似氧化策略的改进。伯和仲非氟化醇都可以高收率被氧化。
更新日期:2018-06-05
中文翻译:

通过氧铵阳离子的结合和光氧化还原催化 对α-三氟甲基和非氟化醇的氧化†
我们提出了一种醇氧化策略,以访问α-三氟甲基酮(TFMKs)合并可见光氧化还原催化的催化氧代铵阳离子氧化。这项工作使用4-乙酰氨基-(2,2,6,6-四甲基-哌啶-1-基)氧基作为有机氧化剂,能够以高收率产生TFMK。该方法是对先前报道的需要超化学计量量的氧化剂的类似氧化策略的改进。伯和仲非氟化醇都可以高收率被氧化。






































 京公网安备 11010802027423号
京公网安备 11010802027423号