当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Model of the Oxygen Evolving Complex Which Is Highly Predisposed to O–O Bond Formation
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2018-06-04 00:00:00 , DOI: 10.1021/acs.jpclett.8b00800 Yulia Pushkar 1 , Katherine M. Davis 2 , Mark C. Palenik 3
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2018-06-04 00:00:00 , DOI: 10.1021/acs.jpclett.8b00800 Yulia Pushkar 1 , Katherine M. Davis 2 , Mark C. Palenik 3
Affiliation
|
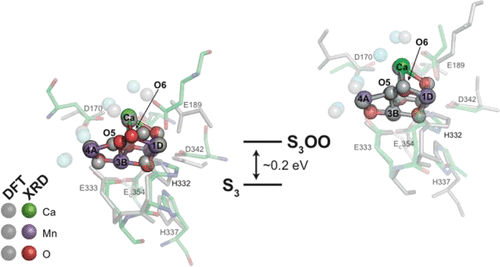
Light-driven water oxidation is a fundamental reaction in the biosphere. The Mn4Ca cluster of photosystem II cycles through five redox states termed S0–S4, after which oxygen is evolved. Critically, the timing of O–O bond formation within the Kok cycle remains unknown. By combining recent crystallographic, spectroscopic, and DFT results, we demonstrate an atomistic S3 state model with the possibility of a low barrier to O–O bond formation prior to the final oxidation step. Furthermore, the associated one electron oxidized S4 state does not provide more advantages in terms of spin alignment or the energy of O–O bond formation. We propose that a high energy peroxide isoform of the S3 state can preferentially be oxidized by Tyrzox in the course of final electron transfer leading to O2 evolution. Such a mechanism may explain the peculiar kinetic behavior of O2 evolution as well as serve as an evolutionary adaptation to avoid release of the harmful peroxides.
中文翻译:

高度倾向于O–O键形成的析氧复合物模型
光驱动的水氧化是生物圈中的基本反应。光系统II的Mn 4 Ca团簇循环通过五个称为S 0 –S 4的氧化还原状态,然后释放出氧气。至关重要的是,在Kok循环中O-O键形成的时机仍然未知。通过结合最新的晶体学,光谱学和DFT结果,我们证明了原子态S 3状态模型,并且在最终氧化步骤之前,对O-O键形成的势垒可能较低。此外,在自旋对准或O-O键形成的能量方面,一个相关的电子被氧化的S 4状态没有提供更多的优势。我们提出S 3的高能过氧化物同工型在最终的电子转移过程中,优先地被Tyr z ox氧化态,导致O 2析出。这种机制可以解释O 2析出的特殊动力学行为,并可以作为进化适应措施,避免有害过氧化物的释放。
更新日期:2018-06-04
中文翻译:

高度倾向于O–O键形成的析氧复合物模型
光驱动的水氧化是生物圈中的基本反应。光系统II的Mn 4 Ca团簇循环通过五个称为S 0 –S 4的氧化还原状态,然后释放出氧气。至关重要的是,在Kok循环中O-O键形成的时机仍然未知。通过结合最新的晶体学,光谱学和DFT结果,我们证明了原子态S 3状态模型,并且在最终氧化步骤之前,对O-O键形成的势垒可能较低。此外,在自旋对准或O-O键形成的能量方面,一个相关的电子被氧化的S 4状态没有提供更多的优势。我们提出S 3的高能过氧化物同工型在最终的电子转移过程中,优先地被Tyr z ox氧化态,导致O 2析出。这种机制可以解释O 2析出的特殊动力学行为,并可以作为进化适应措施,避免有害过氧化物的释放。































 京公网安备 11010802027423号
京公网安备 11010802027423号