当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
MBNL1-mediated regulation of differentiation RNAs promotes myofibroblast transformation and the fibrotic response.
Nature Communications ( IF 14.7 ) Pub Date : 2015-Dec-16 , DOI: 10.1038/ncomms10084
Jennifer Davis , Nathan Salomonis , Natasha Ghearing , Suh-Chin J. Lin , Jennifer Q. Kwong , Apoorva Mohan , Maurice S. Swanson , Jeffery D. Molkentin
Nature Communications ( IF 14.7 ) Pub Date : 2015-Dec-16 , DOI: 10.1038/ncomms10084
Jennifer Davis , Nathan Salomonis , Natasha Ghearing , Suh-Chin J. Lin , Jennifer Q. Kwong , Apoorva Mohan , Maurice S. Swanson , Jeffery D. Molkentin
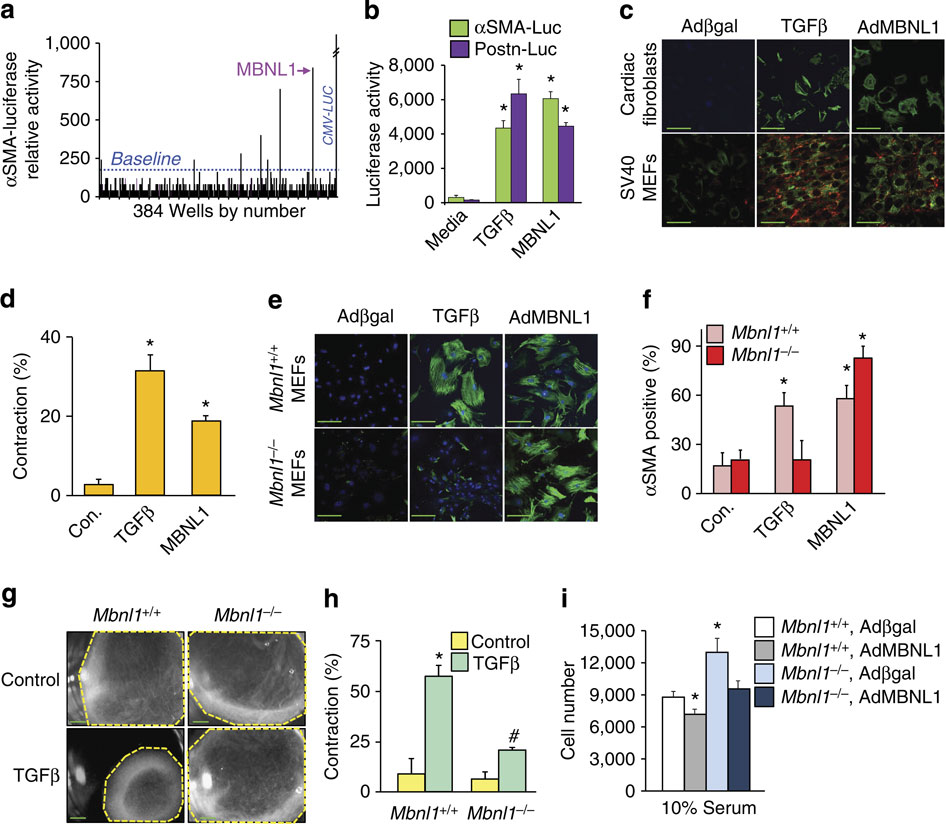
|
The differentiation of fibroblasts into myofibroblasts mediates tissue wound healing and fibrotic remodelling, although the molecular programme underlying this process remains poorly understood. Here we perform a genome-wide screen for genes that control myofibroblast transformation, and identify the RNA-binding protein muscleblind-like1 (MBNL1). MBNL1 overexpression promotes transformation of fibroblasts into myofibroblasts, whereas loss of Mbnl1 abrogates transformation and impairs the fibrotic phase of wound healing in mouse models of myocardial infarction and dermal injury. Mechanistically, MBNL1 directly binds to and regulates a network of differentiation-specific and cytoskeletal/matrix-assembly transcripts to promote myofibroblast differentiation. One of these transcripts is the nodal transcriptional regulator serum response factor (SRF), whereas another is calcineurin Aβ. CRISPR-Cas9-mediated gene-editing of the MBNL1-binding site within the Srf 3'UTR impairs myofibroblast differentiation, whereas in vivo deletion of Srf in fibroblasts impairs wound healing and fibrosis. These data establish a new RNA-dependent paradigm for myofibroblast formation through MBNL1.
中文翻译:

MBNL1介导的分化RNA调控促进成肌纤维细胞转化和纤维化反应。
成纤维细胞向成肌纤维细胞的分化介导了组织伤口的愈合和纤维化重塑,尽管对该过程所依据的分子程序仍知之甚少。在这里,我们对控制成肌纤维细胞转化的基因进行了全基因组筛选,并确定了RNA结合蛋白Muskinblind-like1(MBNL1)。MBNL1的过表达促进成纤维细胞转化为成肌纤维细胞,而Mbnl1的丧失则消除转化并损害心肌梗塞和皮肤损伤小鼠模型中伤口愈合的纤维化阶段。从机制上讲,MBNL1直接结合并调节分化特异性和细胞骨架/基质组装转录本的网络,以促进成肌纤维细胞分化。这些转录物之一是淋巴结转录调节因子血清反应因子(SRF),而另一个是钙调神经磷酸酶Aβ。CRISPR-Cas9介导的Srf 3'UTR中MBNL1结合位点的基因编辑会损害成肌纤维细胞的分化,而体内成纤维细胞中Srf的缺失会损害伤口的愈合和纤维化。这些数据为通过MBNL1形成肌成纤维细胞建立了一种新的依赖RNA的范例。
更新日期:2015-12-19
中文翻译:

MBNL1介导的分化RNA调控促进成肌纤维细胞转化和纤维化反应。
成纤维细胞向成肌纤维细胞的分化介导了组织伤口的愈合和纤维化重塑,尽管对该过程所依据的分子程序仍知之甚少。在这里,我们对控制成肌纤维细胞转化的基因进行了全基因组筛选,并确定了RNA结合蛋白Muskinblind-like1(MBNL1)。MBNL1的过表达促进成纤维细胞转化为成肌纤维细胞,而Mbnl1的丧失则消除转化并损害心肌梗塞和皮肤损伤小鼠模型中伤口愈合的纤维化阶段。从机制上讲,MBNL1直接结合并调节分化特异性和细胞骨架/基质组装转录本的网络,以促进成肌纤维细胞分化。这些转录物之一是淋巴结转录调节因子血清反应因子(SRF),而另一个是钙调神经磷酸酶Aβ。CRISPR-Cas9介导的Srf 3'UTR中MBNL1结合位点的基因编辑会损害成肌纤维细胞的分化,而体内成纤维细胞中Srf的缺失会损害伤口的愈合和纤维化。这些数据为通过MBNL1形成肌成纤维细胞建立了一种新的依赖RNA的范例。

































 京公网安备 11010802027423号
京公网安备 11010802027423号