当前位置:
X-MOL 学术
›
Cell Discov.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cryo-EM structure of the ASIC1a-mambalgin-1 complex reveals that the peptide toxin mambalgin-1 inhibits acid-sensing ion channels through an unusual allosteric effect.
Cell Discovery ( IF 13.0 ) Pub Date : 2018-01-01 , DOI: 10.1038/s41421-018-0026-1 Demeng Sun , You Yu , Xiaobin Xue , Man Pan , Ming Wen , Siyu Li , Qian Qu , Xiaorun Li , Longhua Zhang , Xueming Li , Lei Liu , Maojun Yang , Changlin Tian
Cell Discovery ( IF 13.0 ) Pub Date : 2018-01-01 , DOI: 10.1038/s41421-018-0026-1 Demeng Sun , You Yu , Xiaobin Xue , Man Pan , Ming Wen , Siyu Li , Qian Qu , Xiaorun Li , Longhua Zhang , Xueming Li , Lei Liu , Maojun Yang , Changlin Tian
|
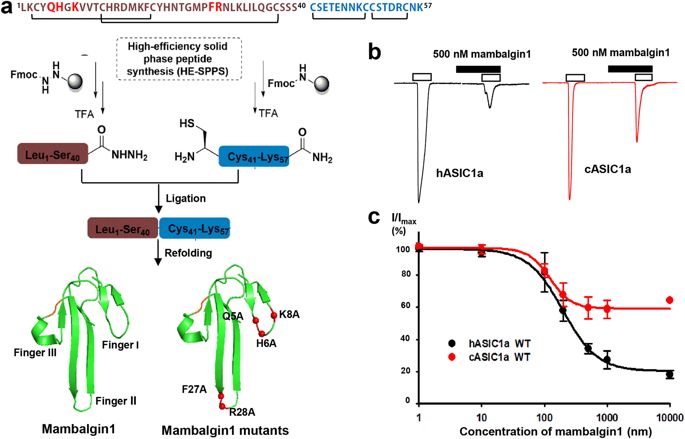
Acid-sensing ion channels (ASICs) are neuronal voltage-independent Na+ channels that are activated by extracellular acidification. ASICs play essential roles in a wide range of physiological processes, including sodium homeostasis, synaptic plasticity, neurodegeneration, and sensory transduction. Mambalgins, a family of three-finger toxins isolated from black mamba venom, specifically inhibit ASICs to exert strong analgesic effects in vivo, thus are thought to have potential therapeutic values against pain. However, the interaction and inhibition mechanism of mambalgin on ASICs remains elusive. Here, we report a cryo-electron microscopy (cryo-EM) structure of chicken ASIC1a (cASIC1a) in complex with mambalgin-1 toxin at 5.4 Å resolution. Our structure provides the first experimental evidence that mambalgin-1 interacts directly with the extracellular thumb domain of cASIC1a, rather than inserting into the acid-sensing pocket, as previously reported. Binding of mambalgin-1 leads to relocation of the thumb domain that could disrupt the acidic pocket of cASIC1a, illustrating an unusual inhibition mechanism of toxins on ASIC channels through an allosteric effect. These findings establish a structural basis for the toxicity of the mambalgins, and provide crucial insights for the development of new optimized inhibitors of ASICs.
中文翻译:

ASIC1a-mambalgin-1复合物的Cryo-EM结构表明肽毒素mambalgin-1通过异常的变构作用抑制酸敏感离子通道。
酸感应离子通道(ASICs)是神经元电压独立的Na +细胞外酸化激活的通道。ASIC在广泛的生理过程中起着至关重要的作用,包括钠稳态,突触可塑性,神经变性和感觉传导。从黑曼巴蛇毒中分离出的三指毒素家族Mambalgins特异地抑制ASICs在体内发挥强镇痛作用,因此被认为具有潜在的止痛治疗价值。但是,mmbalgin在ASIC上的相互作用和抑制机制仍然难以捉摸。在这里,我们报告了鸡ASIC1a(cASIC1a)与mambalgin-1毒素的复合体在5.4Å分辨率下的低温电子显微镜(cryo-EM)结构。我们的结构提供了第一个实验证据,表明mambalgin-1与cASIC1a的细胞外拇指结构域直接相互作用,而不是像以前报道的那样插入酸感应袋中。mambalgin-1的结合导致拇指结构域的重新定位,这可能会破坏cASIC1a的酸性囊袋,这说明通过变构效应,毒素对ASIC通道的异常抑制机制。这些发现为曼巴霉素的毒性奠定了结构基础,并为开发新型优化的ASIC抑制剂提供了重要的见识。
更新日期:2019-01-26
中文翻译:

ASIC1a-mambalgin-1复合物的Cryo-EM结构表明肽毒素mambalgin-1通过异常的变构作用抑制酸敏感离子通道。
酸感应离子通道(ASICs)是神经元电压独立的Na +细胞外酸化激活的通道。ASIC在广泛的生理过程中起着至关重要的作用,包括钠稳态,突触可塑性,神经变性和感觉传导。从黑曼巴蛇毒中分离出的三指毒素家族Mambalgins特异地抑制ASICs在体内发挥强镇痛作用,因此被认为具有潜在的止痛治疗价值。但是,mmbalgin在ASIC上的相互作用和抑制机制仍然难以捉摸。在这里,我们报告了鸡ASIC1a(cASIC1a)与mambalgin-1毒素的复合体在5.4Å分辨率下的低温电子显微镜(cryo-EM)结构。我们的结构提供了第一个实验证据,表明mambalgin-1与cASIC1a的细胞外拇指结构域直接相互作用,而不是像以前报道的那样插入酸感应袋中。mambalgin-1的结合导致拇指结构域的重新定位,这可能会破坏cASIC1a的酸性囊袋,这说明通过变构效应,毒素对ASIC通道的异常抑制机制。这些发现为曼巴霉素的毒性奠定了结构基础,并为开发新型优化的ASIC抑制剂提供了重要的见识。

































 京公网安备 11010802027423号
京公网安备 11010802027423号