当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Telechelic Poly(p-benzamide)s
Macromolecules ( IF 5.1 ) Pub Date : 2018-06-01 00:00:00 , DOI: 10.1021/acs.macromol.8b00587
Mahshid Alizadeh 1 , Andreas F. M. Kilbinger 1
Macromolecules ( IF 5.1 ) Pub Date : 2018-06-01 00:00:00 , DOI: 10.1021/acs.macromol.8b00587
Mahshid Alizadeh 1 , Andreas F. M. Kilbinger 1
Affiliation
|
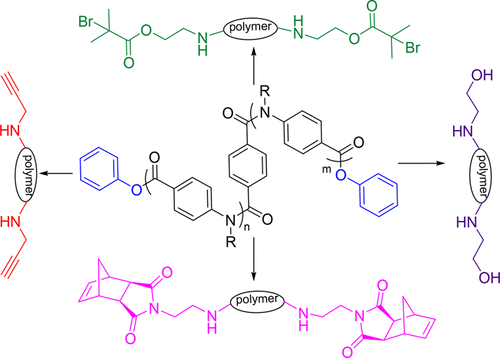
Well-defined telechelic poly(benzamide)s were synthesized by chain-growth polycondensation of phenyl-4-amino benzoate and pentafluorophenyl-4-amino benzoate derivatives with a bifunctional initiator in the presence of LiTMP as base. The polymerization was carried out at −70 °C to prevent self-initiated polymerization. To confirm the control over molecular weight, different defined molecular weight polymers were synthesized and analyzed by GPC. Taking advantage of the labile ester end groups of these poly(benzamide)s, we carried out postpolymerization modifications to introduce different end functional groups such as alkyne, amine, alcohol, alkyl halide, and olefin suitable for different types of postpolymerization reactions. Successful end group modification was confirmed by 1H NMR spectroscopy and isotopically resolved MALDI-ToF mass spectrometry.
中文翻译:

远螯聚(对-苯甲酰胺)s的合成
在LiTMP为碱的情况下,通过双官能引发剂对苯基-4-氨基苯甲酸酯和五氟苯基-4-氨基苯甲酸酯衍生物进行链增长缩聚反应,可以合成定义明确的远螯聚苯甲酰胺。聚合在-70℃下进行以防止自引发聚合。为了确认对分子量的控制,合成了不同的确定分子量的聚合物,并通过GPC进行了分析。利用这些聚(苯甲酰胺)的不稳定酯端基,我们进行了后聚合修饰,以引入适合不同类型的后聚合反应的不同端基官能团,如炔烃,胺,醇,卤代烷和烯烃。成功的端基修饰被1证实1 H NMR光谱法和同位素分辨的MALDI-ToF质谱法。
更新日期:2018-06-01
中文翻译:

远螯聚(对-苯甲酰胺)s的合成
在LiTMP为碱的情况下,通过双官能引发剂对苯基-4-氨基苯甲酸酯和五氟苯基-4-氨基苯甲酸酯衍生物进行链增长缩聚反应,可以合成定义明确的远螯聚苯甲酰胺。聚合在-70℃下进行以防止自引发聚合。为了确认对分子量的控制,合成了不同的确定分子量的聚合物,并通过GPC进行了分析。利用这些聚(苯甲酰胺)的不稳定酯端基,我们进行了后聚合修饰,以引入适合不同类型的后聚合反应的不同端基官能团,如炔烃,胺,醇,卤代烷和烯烃。成功的端基修饰被1证实1 H NMR光谱法和同位素分辨的MALDI-ToF质谱法。

































 京公网安备 11010802027423号
京公网安备 11010802027423号