当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational Analysis of the Intramolecular Oxidative Amination of an Alkene Catalyzed by the Extreme π-Loading N-Heterocyclic Carbene Pincer Tantalum(V) Bis(imido) Complex
Organometallics ( IF 2.5 ) Pub Date : 2018-06-01 , DOI: 10.1021/acs.organomet.8b00097 Guangchao Liang 1 , T. Keith Hollis 1 , Charles Edwin Webster 1
Organometallics ( IF 2.5 ) Pub Date : 2018-06-01 , DOI: 10.1021/acs.organomet.8b00097 Guangchao Liang 1 , T. Keith Hollis 1 , Charles Edwin Webster 1
Affiliation
|
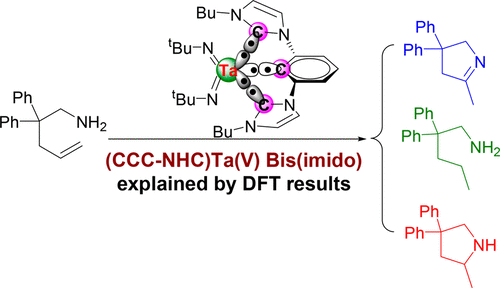
The extreme π-loading of a (CCC-NHC) pincer Ta(V) bis(imido) complex was previously reported. This complex catalyzes the cyclization of α,ω-aminoalkenes (2,2-diphenylpent-4-en-1-amine) to produce three different products in varying proportions: cyclic imine from oxidative amination (OA), reduction product (RP) from hydrogenated substrate, and hydroamination (HA) (Helgert, T. R. ; Organometallics 2016, 35, 3452). Various plausible pathways for the reactions generating cyclic imine product from oxidative amination, the reduction of substrate from hydrogen transfer, the cyclic amine product from hydroamination, and the dehydrogenation of hydroamination product were evaluated using density functional theory computations. RP is the thermodynamic product, while OA and HA are the kinetic products, with HA being lower in energy than OA. Multiple pathways for the generation of OA product were examined. The lowest free energy of activation (ΔG⧧) of the rate-determining-step (RDS) during the oxidative amination was calculated to be 28.8 kcal mol–1. The ΔG⧧ of the RDS for the generation of reduction product is 42.8 kcal mol–1and for the generation of hydroamination product is 41.8 kcal mol–1. The overall turnover-limiting step of the proposed catalytic cycle of the conversions of substrate 3 is the regeneration of the Ta(V) bis(imido) intermediate 7 from Ta(V)-hydride amido intermediate 13 (42.8 kcal mol–1, TS-19-7). An amido hydride Ta intermediate 13 is the computed resting state of the proposed catalytic cycle. High temperature significantly favored the formation of OA-4 and RP-5 and also promoted the dehydrogenation of HA-6. An alternative for the generation of OA-4 with the participation of the NHC as a proton shuttle and through a σN-π-σC isomerization pathway is also discussed. The computational results are consistent with the experimentally observed product ratios and selectivity.
中文翻译:

极端π负载的N-杂环碳原子钳型钽(V)双(亚氨基)配合物催化的烯烃分子内氧化胺的计算分析
以前曾报道过(CCC-NHC)夹钳Ta(V)双(酰亚胺)络合物的极端π负载。该络合物催化α,ω-氨基烯烃(2,2-二苯基五-4-en-1-胺)的环化反应,生成不同比例的三种不同产物:氧化胺(OA)生成的环状亚胺,氧化胺(OA)生成的还原产物(RP)。氢化底物和加氢胺化(HA)(赫尔格特(TR) ; 有机金属化合物2016,35,3452)。使用密度泛函理论计算,评估了氧化胺化反应生成环状亚胺产物,氢转移还原底物,氢化胺化反应生成的环状胺产物以及氢化胺化产物进行脱氢反应的各种可能途径。RP是热力学产物,而OA和HA是动力学产物,其中HA的能量低于OA。检查了产生OA产物的多种途径。活化的最低自由能(Δ ģ ⧧速率决定步骤(RDS)氧化胺化过程中的)计算为28.8千卡摩尔-1。该Δ ģ ⧧RDS的生成还原产物为42.8 kcal mol –1,而氢化胺化产物的生成为41.8 kcal mol –1。提议的底物3转化催化周期的总营业额限制步骤是Ta(V)双(酰亚胺)中间体7从Ta(V)氢化酰胺中间体13(42.8 kcal mol –1,TS -19-7)。酰氨基氢化物Ta中间体13是所提出的催化循环的计算的静止状态。高温显着促进了OA-4和RP-5的形成,也促进了OH-4的脱氢。HA-6。为产生一个替代OA-4与NHC的参与作为质子梭子,并且通过σ Ñ -π-σ Ç还讨论的异构化途径。计算结果与实验观察到的产物比率和选择性一致。
更新日期:2018-06-01
中文翻译:

极端π负载的N-杂环碳原子钳型钽(V)双(亚氨基)配合物催化的烯烃分子内氧化胺的计算分析
以前曾报道过(CCC-NHC)夹钳Ta(V)双(酰亚胺)络合物的极端π负载。该络合物催化α,ω-氨基烯烃(2,2-二苯基五-4-en-1-胺)的环化反应,生成不同比例的三种不同产物:氧化胺(OA)生成的环状亚胺,氧化胺(OA)生成的还原产物(RP)。氢化底物和加氢胺化(HA)(


















































 京公网安备 11010802027423号
京公网安备 11010802027423号