当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interaction of Arsenous Acid with the Dithiol-Type Chelator British Anti-Lewisite (BAL): Structure and Stability of Species Formed in an Unexpectedly Complex System
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-06-01 00:00:00 , DOI: 10.1021/acs.inorgchem.8b00894 Levente I. Szekeres 1 , Béla Gyurcsik 1 , Tamás Kiss 1 , Zoltán Kele 2 , Attila Jancsó 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-06-01 00:00:00 , DOI: 10.1021/acs.inorgchem.8b00894 Levente I. Szekeres 1 , Béla Gyurcsik 1 , Tamás Kiss 1 , Zoltán Kele 2 , Attila Jancsó 1
Affiliation
|
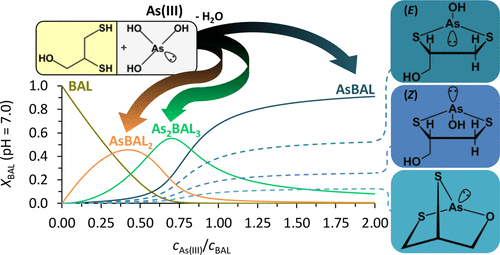
British anti-Lewisite (2,3-dimerkaptopropan-1-ol, dimercaprol, BAL) is one of the best-known chelator-type therapeutic agents against toxic metal ions and metalloids, especially arsenicals. Surprisingly, the mechanisms of action at the molecular level, as well as the coordination features of this traditional drug toward various arsenicals, are still poorly revealed. The present study on the interaction of arsenous acid (H3AsO3) with BAL, involving UV and NMR titrations, electrospray ionization mass spectrometry, and 2D NMR experiments combined with MP2 calculations, demonstrates that the reaction of H3AsO3 with BAL at pH = 7.0 results in a more complex speciation than was assumed before. The three reactive hydroxyl groups of H3AsO3 allow for interaction with three thiol moieties via condensation reaction, leading to the observed AsBAL2 and As2BAL3 complexes besides the AsBAL species. This indicates the strong propensity of inorganic As(III) to saturate its coordination sphere with thiolate groups. The alcoholic hydroxyl group of the ligand may also directly bind to As(III) in AsBAL. Compared to dithiothreitol or dithioeritritol, the preference of BAL to form complexes with such a tridentate binding mode is much lower owing to the more strained bridged bicyclic structure with an αAsSC < 90° bond angle and an unfavorable condensed boat-type six-membered ring. On the basis of the NMR data, the predominating, bidentately bound AsBAL species, including a five-membered chelate ring, exists in rapidly interconverting envelope forms of E and Z stereoisomers. The conditional stability constants calculated for the three macrospecies from a series of UV data [log βpH=7.0 = 6.95 (AsBAL), 11.56 (AsBAL2), and 22.73 (As2BAL3)] reflect that BAL is still the most efficient, known, dithiol-type chelator of H3AsO3.
中文翻译:

亚砷酸与二硫醇型螯合剂英国反路易斯(BAL)的相互作用:意外复杂的系统中形成的物种的结构和稳定性。
英国抗路易斯碱(2,3-dimerkaptopropan-1-ol,dimercaprol,BAL)是针对有毒金属离子和准金属(尤其是砷化物)的最著名的螯合剂型治疗剂之一。令人惊讶的是,在分子水平上的作用机理以及这种传统药物对各种砷的配位特征仍然鲜为人知。目前关于亚砷酸(H 3 AsO 3)与BAL相互作用的研究,包括UV和NMR滴定,电喷雾电离质谱,二维NMR实验以及MP2计算,证明了H 3 AsO 3与BAL的反应在pH = 7.0导致形成的物种比以前假设的更为复杂。H的三个反应性羟基3 AsO 3允许通过缩合反应与三个巯基部分相互作用,从而导致除了AsBAL物种外还观察到AsBAL 2和As 2 BAL 3络合物。这表明无机As(III)强烈倾向于将其配位体与硫醇盐基团一起饱和。配体的醇羟基也可以直接与AsBAL中的As(III)结合。与二硫苏糖醇或二硫赤藓糖醇相比,由于具有更强的αAsSC桥联双环结构,BAL选择具有这种三齿结合模式的复合物的偏好要低得多。<90°键角和不利的冷凝式船形六元环。根据NMR数据,包括五元螯合环在内的占主导地位的,二齿结合的AsBAL物质以快速相互转化的E和Z立体异构体的包膜形式存在。根据一系列紫外线数据[ logβpH = 7.0 = 6.95(AsBAL),11.56(AsBAL 2)和22.73(As 2 BAL 3)]为这三个大种计算的条件稳定性常数反映出BAL仍然是最有效的,已知的H 3 AsO 3的二硫醇型螯合剂。
更新日期:2018-06-01
中文翻译:

亚砷酸与二硫醇型螯合剂英国反路易斯(BAL)的相互作用:意外复杂的系统中形成的物种的结构和稳定性。
英国抗路易斯碱(2,3-dimerkaptopropan-1-ol,dimercaprol,BAL)是针对有毒金属离子和准金属(尤其是砷化物)的最著名的螯合剂型治疗剂之一。令人惊讶的是,在分子水平上的作用机理以及这种传统药物对各种砷的配位特征仍然鲜为人知。目前关于亚砷酸(H 3 AsO 3)与BAL相互作用的研究,包括UV和NMR滴定,电喷雾电离质谱,二维NMR实验以及MP2计算,证明了H 3 AsO 3与BAL的反应在pH = 7.0导致形成的物种比以前假设的更为复杂。H的三个反应性羟基3 AsO 3允许通过缩合反应与三个巯基部分相互作用,从而导致除了AsBAL物种外还观察到AsBAL 2和As 2 BAL 3络合物。这表明无机As(III)强烈倾向于将其配位体与硫醇盐基团一起饱和。配体的醇羟基也可以直接与AsBAL中的As(III)结合。与二硫苏糖醇或二硫赤藓糖醇相比,由于具有更强的αAsSC桥联双环结构,BAL选择具有这种三齿结合模式的复合物的偏好要低得多。<90°键角和不利的冷凝式船形六元环。根据NMR数据,包括五元螯合环在内的占主导地位的,二齿结合的AsBAL物质以快速相互转化的E和Z立体异构体的包膜形式存在。根据一系列紫外线数据[ logβpH = 7.0 = 6.95(AsBAL),11.56(AsBAL 2)和22.73(As 2 BAL 3)]为这三个大种计算的条件稳定性常数反映出BAL仍然是最有效的,已知的H 3 AsO 3的二硫醇型螯合剂。

































 京公网安备 11010802027423号
京公网安备 11010802027423号