当前位置:
X-MOL 学术
›
ACS Biomater. Sci. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism of Antibacterial Activity of Choline-Based Ionic Liquids (CAGE)
ACS Biomaterials Science & Engineering ( IF 5.4 ) Pub Date : 2018-05-31 00:00:00 , DOI: 10.1021/acsbiomaterials.8b00486 Kelly N. Ibsen 1, 2 , Huilin Ma 3 , Amrita Banerjee 1 , Eden E. L. Tanner 2 , Shikha Nangia 3 , Samir Mitragotri 2
ACS Biomaterials Science & Engineering ( IF 5.4 ) Pub Date : 2018-05-31 00:00:00 , DOI: 10.1021/acsbiomaterials.8b00486 Kelly N. Ibsen 1, 2 , Huilin Ma 3 , Amrita Banerjee 1 , Eden E. L. Tanner 2 , Shikha Nangia 3 , Samir Mitragotri 2
Affiliation
|
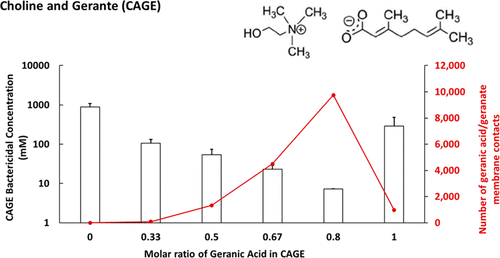
The continued emergence of antibiotic-resistant organisms has severely depleted our arsenal of effective antimicrobials. Ionic liquids (ILs) show great promise as antibacterial agents but understanding the mechanism of attack on bacterial cells is key to ensuring that design of IL-based biocides impart maximum efficacy with minimal toxicity, while also avoiding the potential for the target organisms to become resistant. Here we report the antibacterial attributes of a set of choline and geranate (CAGE)-based ILs and identify the mechanism by which they interact with the Gram-negative cell wall of Escherichia coli. CAGE is envisaged as an antimicrobial agent to treat topical infections in skin. Our earlier work has shown that CAGE is highly effective across a breadth of bacterial, fungal, and viral species and is benign to human cells. This combination makes CAGE an ideal antimicrobial for human use. Four CAGE variants with varying ratios of choline and geranic acid were synthesized and tested for their antibacterial activity (1:4, 1:2, 1:1, and 2:1 choline:geranic acid). The minimum bactericidal concentration required to kill E. coli correlated with the geranic acid content. Using molecular dynamics (MD) simulations, we identified the mechanism of CAGE action on the E. coli membrane, namely that choline is attracted to the negatively charged cell membrane and consequently inserts geranic acid into the lipid bilayer. The disruption of the cell membrane was confirmed with propidium iodide staining via flow cytometry and scanning electron microscopy. Fourier Transform infrared spectroscopic analysis of treated cells showed an altered lipid profile similar to phase transition, indicating the disruption of the lipid bilayer conformation. E. coli cells repeatedly exposed to CAGE did not exhibit resistance. This study provides the fundamental mechanism of the action of choline-based ILs on Gram-negative bacteria and demonstrates the promise of CAGE as a powerful antimicrobial agent to treat infections.
中文翻译:

胆碱离子液体(CAGE)的抗菌活性机理
抗生素耐药性微生物的不断出现严重消耗了我们有效抗菌药物的库。离子液体(ILs)作为抗菌剂显示出广阔的前景,但了解对细菌细胞的攻击机制是确保基于IL的生物杀伤剂的设计能够以最小的毒性发挥最大功效的关键,同时还避免了靶标生物产生耐药性的可能性。在这里,我们报告一组基于胆碱和geranate(CAGE)的IL的抗菌特性,并确定它们与大肠杆菌革兰氏阴性细胞壁相互作用的机制。设想将CAGE用作治疗皮肤局部感染的抗菌剂。我们的早期工作表明,CAGE在多种细菌,真菌和病毒物种中非常有效,并且对人体细胞无害。这种组合使CAGE成为人类理想的抗菌剂。合成了胆碱和香叶酸比例不同的四个CAGE变体,并测试了它们的抗菌活性(1:4、1:2、1:1和2:1胆碱:香叶酸)。杀死大肠杆菌所需的最低杀菌浓度与香叶酸含量有关。使用分子动力学(MD)模拟,我们确定了CAGE对大肠杆菌的作用机理膜,即胆碱被吸引到带负电荷的细胞膜上,因此将香叶酸插入脂质双层中。通过流式细胞术和扫描电子显微镜用碘化丙啶染色证实细胞膜的破坏。经处理的细胞的傅立叶变换红外光谱分析表明,脂质的变化与相变相似,表明脂质双层构象被破坏。反复暴露于CAGE的大肠杆菌细胞不显示抗药性。这项研究提供了基于胆碱的ILs对革兰氏阴性细菌起作用的基本机制,并证明了CAGE作为治疗感染的强大抗菌剂的前景。
更新日期:2018-05-31
中文翻译:

胆碱离子液体(CAGE)的抗菌活性机理
抗生素耐药性微生物的不断出现严重消耗了我们有效抗菌药物的库。离子液体(ILs)作为抗菌剂显示出广阔的前景,但了解对细菌细胞的攻击机制是确保基于IL的生物杀伤剂的设计能够以最小的毒性发挥最大功效的关键,同时还避免了靶标生物产生耐药性的可能性。在这里,我们报告一组基于胆碱和geranate(CAGE)的IL的抗菌特性,并确定它们与大肠杆菌革兰氏阴性细胞壁相互作用的机制。设想将CAGE用作治疗皮肤局部感染的抗菌剂。我们的早期工作表明,CAGE在多种细菌,真菌和病毒物种中非常有效,并且对人体细胞无害。这种组合使CAGE成为人类理想的抗菌剂。合成了胆碱和香叶酸比例不同的四个CAGE变体,并测试了它们的抗菌活性(1:4、1:2、1:1和2:1胆碱:香叶酸)。杀死大肠杆菌所需的最低杀菌浓度与香叶酸含量有关。使用分子动力学(MD)模拟,我们确定了CAGE对大肠杆菌的作用机理膜,即胆碱被吸引到带负电荷的细胞膜上,因此将香叶酸插入脂质双层中。通过流式细胞术和扫描电子显微镜用碘化丙啶染色证实细胞膜的破坏。经处理的细胞的傅立叶变换红外光谱分析表明,脂质的变化与相变相似,表明脂质双层构象被破坏。反复暴露于CAGE的大肠杆菌细胞不显示抗药性。这项研究提供了基于胆碱的ILs对革兰氏阴性细菌起作用的基本机制,并证明了CAGE作为治疗感染的强大抗菌剂的前景。






























 京公网安备 11010802027423号
京公网安备 11010802027423号