Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Acid Suspends the Circadian Clock in Hypoxia through Inhibition of mTOR.
Cell ( IF 45.5 ) Pub Date : 2018-Jun-28 , DOI: 10.1016/j.cell.2018.05.009 Zandra E Walton 1 , Chirag H Patel 2 , Rebekah C Brooks 1 , Yongjun Yu 1 , Arig Ibrahim-Hashim 3 , Malini Riddle 4 , Alessandra Porcu 4 , Tianying Jiang 5 , Brett L Ecker 6 , Feven Tameire 7 , Constantinos Koumenis 7 , Ashani T Weeraratna 5 , David K Welsh 4 , Robert Gillies 3 , James C Alwine 1 , Lin Zhang 8 , Jonathan D Powell 2 , Chi V Dang 9
Cell ( IF 45.5 ) Pub Date : 2018-Jun-28 , DOI: 10.1016/j.cell.2018.05.009 Zandra E Walton 1 , Chirag H Patel 2 , Rebekah C Brooks 1 , Yongjun Yu 1 , Arig Ibrahim-Hashim 3 , Malini Riddle 4 , Alessandra Porcu 4 , Tianying Jiang 5 , Brett L Ecker 6 , Feven Tameire 7 , Constantinos Koumenis 7 , Ashani T Weeraratna 5 , David K Welsh 4 , Robert Gillies 3 , James C Alwine 1 , Lin Zhang 8 , Jonathan D Powell 2 , Chi V Dang 9
Affiliation
|
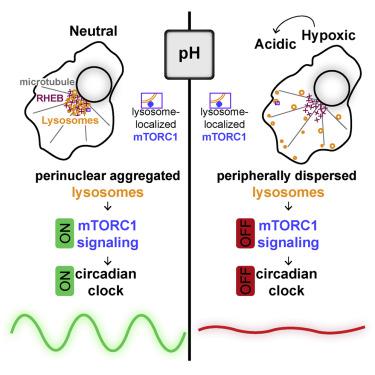
Recent reports indicate that hypoxia influences the circadian clock through the transcriptional activities of hypoxia-inducible factors (HIFs) at clock genes. Unexpectedly, we uncover a profound disruption of the circadian clock and diurnal transcriptome when hypoxic cells are permitted to acidify to recapitulate the tumor microenvironment. Buffering against acidification or inhibiting lactic acid production fully rescues circadian oscillation. Acidification of several human and murine cell lines, as well as primary murine T cells, suppresses mechanistic target of rapamycin complex 1 (mTORC1) signaling, a key regulator of translation in response to metabolic status. We find that acid drives peripheral redistribution of normally perinuclear lysosomes away from perinuclear RHEB, thereby inhibiting the activity of lysosome-bound mTOR. Restoring mTORC1 signaling and the translation it governs rescues clock oscillation. Our findings thus reveal a model in which acid produced during the cellular metabolic response to hypoxia suppresses the circadian clock through diminished translation of clock constituents.
中文翻译:

酸通过抑制 mTOR 使缺氧状态下的生物钟暂停。
最近的报告表明,缺氧通过时钟基因缺氧诱导因子(HIF)的转录活性影响生物钟。出乎意料的是,我们发现当缺氧细胞被酸化以重现肿瘤微环境时,生物钟和昼夜转录组会发生严重破坏。缓冲酸化或抑制乳酸产生可以完全挽救昼夜节律振荡。几种人类和鼠类细胞系以及原代鼠类 T 细胞的酸化会抑制雷帕霉素复合物 1 (mTORC1) 信号传导的机制靶标,这是响应代谢状态的翻译的关键调节因子。我们发现酸驱动正常核周溶酶体的外周重新分布远离核周 RHEB,从而抑制溶酶体结合的 mTOR 的活性。恢复 mTORC1 信号传导及其控制的翻译可挽救时钟振荡。因此,我们的研究结果揭示了一个模型,其中细胞对缺氧的代谢反应过程中产生的酸通过减少时钟成分的翻译来抑制生物钟。
更新日期:2018-05-31
中文翻译:

酸通过抑制 mTOR 使缺氧状态下的生物钟暂停。
最近的报告表明,缺氧通过时钟基因缺氧诱导因子(HIF)的转录活性影响生物钟。出乎意料的是,我们发现当缺氧细胞被酸化以重现肿瘤微环境时,生物钟和昼夜转录组会发生严重破坏。缓冲酸化或抑制乳酸产生可以完全挽救昼夜节律振荡。几种人类和鼠类细胞系以及原代鼠类 T 细胞的酸化会抑制雷帕霉素复合物 1 (mTORC1) 信号传导的机制靶标,这是响应代谢状态的翻译的关键调节因子。我们发现酸驱动正常核周溶酶体的外周重新分布远离核周 RHEB,从而抑制溶酶体结合的 mTOR 的活性。恢复 mTORC1 信号传导及其控制的翻译可挽救时钟振荡。因此,我们的研究结果揭示了一个模型,其中细胞对缺氧的代谢反应过程中产生的酸通过减少时钟成分的翻译来抑制生物钟。































 京公网安备 11010802027423号
京公网安备 11010802027423号