当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism and Kinetics of Hydrogen Peroxide Decomposition on Platinum Nanocatalysts
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2018-05-31 00:00:00 , DOI: 10.1021/acsami.8b02345 Rui Serra-Maia , Marion Bellier , Stephen Chastka , Kevin Tranhuu , Andrew Subowo , J. Donald Rimstidt , Pavel M. Usov , Amanda J. Morris , F. Marc Michel
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2018-05-31 00:00:00 , DOI: 10.1021/acsami.8b02345 Rui Serra-Maia , Marion Bellier , Stephen Chastka , Kevin Tranhuu , Andrew Subowo , J. Donald Rimstidt , Pavel M. Usov , Amanda J. Morris , F. Marc Michel
|
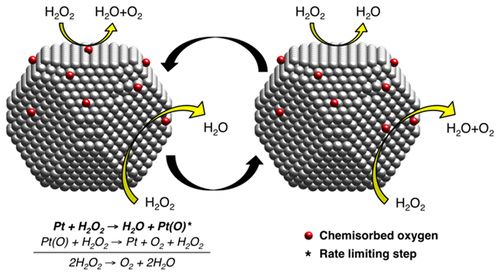
The decomposition of H2O2 to H2O and O2 catalyzed by platinum nanocatalysts controls the energy yield of several energy conversion technologies, such as hydrogen fuel cells. However, the reaction mechanism and rate-limiting step of this reaction have been unsolved for more than 100 years. We determined both the reaction mechanism and rate-limiting step by studying the effect of different reaction conditions, nanoparticle size, and surface composition on the rates of H2O2 decomposition by three platinum nanocatalysts with average particle sizes of 3, 11, and 22 nm. Rate models indicate that the reaction pathway of H2O2 decomposition is similar for all three nanocatalysts. Larger particle size correlates with lower activation energy and enhanced catalytic activity, explained by a smaller work function for larger platinum particles, which favors chemisorption of oxygen onto platinum to form Pt(O). Our experiments also showed that incorporation of oxygen at the nanocatalyst surface results in a faster reaction rate because the rate-limiting step is skipped in the first cycle of reaction. Taken together, these results indicate that the reaction proceeds in two cyclic steps and that step 1 is the rate-limiting step. Step 1: Pt+H2O2 →H2O+Pt(O). Step 2: Pt(O) + H2O2 → Pt + O2 + H2O. Overall: 2H2O2 → O2 + 2H2O. Establishing relationships between the properties of commercial nanocatalysts and their catalytic activity, as we have done here for platinum in the decomposition of H2O2, opens the possibility of improving the performance of nanocatalysts used in applications. This study also demonstrates the advantage of combining detailed characterization and systematic reactivity experiments to understand property–behavior relationships.
中文翻译:

铂纳米催化剂上过氧化氢分解的机理和动力学
铂纳米催化剂催化将H 2 O 2分解为H 2 O和O 2,可控制氢燃料电池等多种能量转换技术的能量产率。但是,该反应的反应机理和限速步骤已经解决了100多年。通过研究不同反应条件,纳米颗粒尺寸和表面组成对三种平均粒径分别为3、11和22的铂纳米催化剂对H 2 O 2分解速率的影响,我们确定了反应机理和限速步骤。纳米 速率模型表明H 2 O 2的反应途径三种纳米催化剂的分解相似。较大的粒径与较低的活化能和增强的催化活性有关,这可通过较大的铂颗粒的较小功函数来解释,这有利于氧化学吸附到铂上以形成Pt(O)。我们的实验还表明,在纳米催化剂表面掺入氧气会导致更快的反应速率,因为在反应的第一个循环中跳过了限速步骤。总而言之,这些结果表明反应以两个循环步骤进行,并且步骤1是限速步骤。步骤1:Pt + H 2 O 2 → H 2 O + Pt(O)。第2步:的Pt(ø)+ ħ 2 ö 2 →铂+ Ò 2 + ħ 2 ö。总体:2 ħ 2 ö 2 → Ò 2 + 2 ħ 2 ö。建立商用纳米催化剂的性质与其催化活性之间的关系,正如我们在此处对H 2 O 2分解中的铂所做的那样,为改善应用中使用的纳米催化剂的性能开辟了可能性。这项研究还证明了将详细的表征和系统的反应性实验相结合以了解特性与行为之间的关系的优势。
更新日期:2018-05-31
中文翻译:

铂纳米催化剂上过氧化氢分解的机理和动力学
铂纳米催化剂催化将H 2 O 2分解为H 2 O和O 2,可控制氢燃料电池等多种能量转换技术的能量产率。但是,该反应的反应机理和限速步骤已经解决了100多年。通过研究不同反应条件,纳米颗粒尺寸和表面组成对三种平均粒径分别为3、11和22的铂纳米催化剂对H 2 O 2分解速率的影响,我们确定了反应机理和限速步骤。纳米 速率模型表明H 2 O 2的反应途径三种纳米催化剂的分解相似。较大的粒径与较低的活化能和增强的催化活性有关,这可通过较大的铂颗粒的较小功函数来解释,这有利于氧化学吸附到铂上以形成Pt(O)。我们的实验还表明,在纳米催化剂表面掺入氧气会导致更快的反应速率,因为在反应的第一个循环中跳过了限速步骤。总而言之,这些结果表明反应以两个循环步骤进行,并且步骤1是限速步骤。步骤1:Pt + H 2 O 2 → H 2 O + Pt(O)。第2步:的Pt(ø)+ ħ 2 ö 2 →铂+ Ò 2 + ħ 2 ö。总体:2 ħ 2 ö 2 → Ò 2 + 2 ħ 2 ö。建立商用纳米催化剂的性质与其催化活性之间的关系,正如我们在此处对H 2 O 2分解中的铂所做的那样,为改善应用中使用的纳米催化剂的性能开辟了可能性。这项研究还证明了将详细的表征和系统的反应性实验相结合以了解特性与行为之间的关系的优势。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号