当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tunable Hydrophobic Eutectic Solvents Based on Terpenes and Monocarboxylic Acids
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2018-05-29 00:00:00 , DOI: 10.1021/acssuschemeng.8b01203 Mónia A. R. Martins 1, 2, 3, 4 , Emanuel A. Crespo 1, 5 , Paula V. A. Pontes 4 , Liliana P. Silva 1 , Mark Bülow 5 , Guilherme J. Maximo 4 , Eduardo A. C. Batista 4 , Christoph Held 5 , Simão P. Pinho 2, 3 , João A. P. Coutinho 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2018-05-29 00:00:00 , DOI: 10.1021/acssuschemeng.8b01203 Mónia A. R. Martins 1, 2, 3, 4 , Emanuel A. Crespo 1, 5 , Paula V. A. Pontes 4 , Liliana P. Silva 1 , Mark Bülow 5 , Guilherme J. Maximo 4 , Eduardo A. C. Batista 4 , Christoph Held 5 , Simão P. Pinho 2, 3 , João A. P. Coutinho 1
Affiliation
|
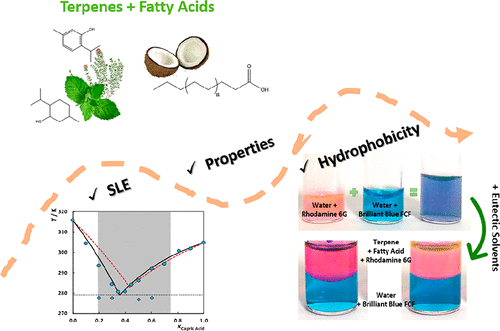
Recently, some works claim that hydrophobic deep eutectic solvents could be prepared based on menthol and monocarboxylic acids. Despite of some promising potential applications, these systems were poorly understood, and this work addresses this issue. Here, the characterization of eutectic solvents composed of the terpenes thymol or l(−)-menthol and monocarboxylic acids is studied aiming the design of these solvents. Their solid–liquid phase diagrams were measured by differential scanning calorimetry in the whole composition range, showing that a broader composition range, and not only fixed stoichiometric proportions, can be used as solvents at low temperatures. Additionally, solvent densities and viscosities close to the eutectic compositions were measured, showing low viscosity and lower density than water. The solvatochromic parameters at the eutectic composition were also investigated aiming at better understanding their polarity. The high acidity is mainly provided by the presence of thymol in the mixture, while l(−)-menthol plays the major role on the hydrogen-bond basicity. The measured mutual solubilities with water attest to the hydrophobic character of the mixtures investigated. The experimental solid–liquid phase diagrams were described using the PC-SAFT equation of state that is shown to accurately describe the experimental data and quantify the small deviations from ideality.
中文翻译:

基于萜烯和一元羧酸的可调节疏水共晶溶剂
最近,一些工作声称可以基于薄荷醇和一元羧酸制备疏水性深共晶溶剂。尽管有一些潜在的潜在应用,但对这些系统的了解却很少,这项工作解决了这个问题。在这里,由萜烯百里香酚或l组成的共晶溶剂的表征针对这些溶剂的设计,对(-)-薄荷醇和一元羧酸进行了研究。通过差示扫描量热法在整个组成范围内测量了它们的固液相图,表明在低温下,更宽的组成范围,不仅是固定的化学计量比,都可以用作溶剂。另外,测量了接近共晶组合物的溶剂密度和粘度,显示出比水低的粘度和低的密度。为了更好地了解它们的极性,还研究了在共晶组成上的溶剂变色参数。高酸度主要由百里酚的在混合物中存在提供,而升(-)-薄荷醇在氢键碱性上起主要作用。所测得的与水的互溶性证明了所研究混合物的疏水性。使用PC-SAFT状态方程描述了实验固液相图,该方程可准确描述实验数据并量化与理想情况之间的细微偏差。
更新日期:2018-05-29
中文翻译:

基于萜烯和一元羧酸的可调节疏水共晶溶剂
最近,一些工作声称可以基于薄荷醇和一元羧酸制备疏水性深共晶溶剂。尽管有一些潜在的潜在应用,但对这些系统的了解却很少,这项工作解决了这个问题。在这里,由萜烯百里香酚或l组成的共晶溶剂的表征针对这些溶剂的设计,对(-)-薄荷醇和一元羧酸进行了研究。通过差示扫描量热法在整个组成范围内测量了它们的固液相图,表明在低温下,更宽的组成范围,不仅是固定的化学计量比,都可以用作溶剂。另外,测量了接近共晶组合物的溶剂密度和粘度,显示出比水低的粘度和低的密度。为了更好地了解它们的极性,还研究了在共晶组成上的溶剂变色参数。高酸度主要由百里酚的在混合物中存在提供,而升(-)-薄荷醇在氢键碱性上起主要作用。所测得的与水的互溶性证明了所研究混合物的疏水性。使用PC-SAFT状态方程描述了实验固液相图,该方程可准确描述实验数据并量化与理想情况之间的细微偏差。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号